Products Categories
| CAS No.: | 49562-28-9 |
|---|---|
| Name: | Fenofibrate |
| Article Data: | 52 |
| Molecular Structure: | |
|
|
|
| Formula: | C20H21ClO4 |
| Molecular Weight: | 360.837 |
| Synonyms: | Fenogal;Elasterin;Liposit;Fenofibrate [BAN:INN];Lipofene;Lipantil;Elasterate;propan-2-yl 2-[4-(4-chlorobenzoyl)phenoxy]-2-methyl-propanoate;Prestwick_217;Propanoic acid,2-[4-(4-chlorobenzoyl)phenoxy]- 2-methyl-,1-methylethyl ester;Protolipan;Luxacor;Isopropyl 2-(p-(p-chlorobenzoyl)phenoxy)-2-methylpropionate;Procetofen;Secalip;Finofibrate;Lipifen;Isopropyl 2-(4-(4-chlorobenzoyl)phenoxy)-2-methylpropionate;Ankebin;Propanoic acid, 2-(4-(4-chlorobenzoyl)phenoxy)-2-methyl-, 1-methylethyl ester;Fenofibratum [INN-Latin];FNF;Fenobrate;Triglide;Lipidil;Fenofibrate (JAN);Nolipax;LF-178;2-(4-(4-Chlorobenzoyl)phenoxy)-2-methylpropanoic acid 1-methylethyl ester;Isopropyl (4-(p-chlorobenzoyl)-2-phenoxy-2-methyl)propionate;Antara;Lipirex;Lipantil (TN);Fenofibrat; |
| EINECS: | 256-376-3 |
| Density: | 1.177 g/cm3 |
| Melting Point: | 80-81 °C |
| Boiling Point: | 469.8 °C at 760 mmHg |
| Flash Point: | 165.4 °C |
| Solubility: | 0.8mg/L(25 oC) |
| Appearance: | Crystalline solid |
| Hazard Symbols: |
 Xn, Xn,  Xi Xi
|
| Risk Codes: | 22-36/37/38 |
| Safety: | 36-26 |
| PSA: | 52.60000 |
| LogP: | 4.68000 |
- 5156-58-1N-(1-Benzyl-4-pipperidinyl)-N-phenylpropanamide HCl
- 81281-59-67-Benzylideneaminotheophylline
- 50288-62-5threo-Phenyl-2-piperidyl acetamide
- 82993-81-5D-threo-Ritalinic acid hydrochloride
- 73441-42-6METHYL-5-CHLORO-2,2-DIMETHYLVALERATE
- 68439-39-4Poly(oxy-1,2-ethanediyl), alpha-(2-ethylhexyl)-omega-hydroxy-,
- 852475-26-4MC1568
- 958254-66-51H-Imidazo[4,5-b]pyridine-2-carboxaldehyde, 1-methyl-, hydrochloride
- 99170-93-1N-Methyl-2-oxazolamine
- 717878-06-31-(4-fluorophenyl)-4-nitro-1H-imidazole

| Conditions | Yield |
|---|---|
| With magneticnanosolidsuperacid In cyclohexane; water at 83 - 85℃; Reagent/catalyst; Temperature; | 97% |
| Stage #1: fenofibric acid; isopropyl alcohol With thionyl chloride for 7h; Reflux; Stage #2: With potassium carbonate In water at 60 - 65℃; | 96.6% |
| With macroporous strong acid cation exchange resin D001 In water; toluene at 110℃; | 92.4% |

| Conditions | Yield |
|---|---|
| Stage #1: fenofibric acid With potassium carbonate In Isopropyl acetate; dimethyl sulfoxide at 20 - 90℃; for 0.75h; Industry scale; Inert atmosphere; Stage #2: isopropyl bromide In Isopropyl acetate; dimethyl sulfoxide at 80 - 95℃; for 5.83333h; | 94.9% |


- 49562-28-9
fenofibrate

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; copper (II) carbonate hydroxide; TPGS-750-M In water at 20℃; Green chemistry; | 91% |

- 51368-55-9
isopropyl 2-bromo-2‑methylpropanoate


- 42019-78-3
4-chloro-4'-hydroxybenzophenone


- 49562-28-9
fenofibrate

| Conditions | Yield |
|---|---|
| With potassium hydrogencarbonate In isopropyl alcohol for 48h; Reflux; | 90% |
| Stage #1: 4-chloro-4'-hydroxybenzophenone With sodium hydroxide In butanone for 1h; Reflux; Stage #2: isopropyl 2-bromo-2‑methylpropanoate In butanone for 8h; Reflux; | 90% |
| Stage #1: 4-chloro-4'-hydroxybenzophenone With potassium hydrogencarbonate In isopropyl alcohol at 25℃; for 0.166667h; Stage #2: isopropyl 2-bromo-2‑methylpropanoate In isopropyl alcohol at 90℃; for 48h; | 90% |

- 1375008-18-6
isopropyl 2-(4-iodophenoxy)-2-methylpropanoate


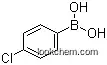
- 1679-18-1
4-Chlorophenylboronic acid


- 13939-06-5, 199620-15-0
molybdenum hexacarbonyl


- 49562-28-9
fenofibrate

| Conditions | Yield |
|---|---|
| With potassium phosphate; palladium(II) trifluoroacetate In water; acetonitrile at 50℃; for 6h; Suzuki Coupling; Inert atmosphere; Sealed tube; | 90% |


- 201230-82-2
carbon monoxide


- 1375008-18-6
isopropyl 2-(4-iodophenoxy)-2-methylpropanoate


- 1679-18-1
4-Chlorophenylboronic acid


- 49562-28-9
fenofibrate

| Conditions | Yield |
|---|---|
| With potassium carbonate; palladium dichloride at 80℃; for 16h; Sealed tube; | 89% |
| With tris-(dibenzylideneacetone)dipalladium(0); potassium carbonate; triethylamine In 1,4-dioxane at 80℃; for 20h; Inert atmosphere; Sealed tube; Cooling with ice; | 80% |
| With potassium carbonate; triphenylphosphine; palladium dichloride at 80℃; for 18h; | 65 %Chromat. |


- 1375008-18-6
isopropyl 2-(4-iodophenoxy)-2-methylpropanoate


- 1679-18-1
4-Chlorophenylboronic acid


- 82102-37-2
9-methyl-9H-fluorene-9-carbonyl chloride


- 49562-28-9
fenofibrate

| Conditions | Yield |
|---|---|
| Stage #1: 9-methyl-9H-fluorene-9-carbonyl chloride With N-ethyl-N,N-diisopropylamine; bis(dibenzylideneacetone)-palladium(0); tri tert-butylphosphoniumtetrafluoroborate at 80℃; Stage #2: isopropyl 2-(4-iodophenoxy)-2-methylpropanoate; 4-Chlorophenylboronic acid With potassium carbonate; palladium dichloride at 80℃; | 84% |


- 1581307-35-8
isopropyl 2-(4-bromophenoxy)-2-methylpropanoate


- 5123-08-0
2-(4-chloro-phenyl)-[1,3,6,2]dioxazaborocane


- 82102-37-2
9-methyl-9H-fluorene-9-carbonyl chloride


- 49562-28-9
fenofibrate

| Conditions | Yield |
|---|---|
| With palladium(II) acetylacetonate; N-ethyl-N,N-diisopropylamine; bis(dibenzylideneacetone)-palladium(0); tri tert-butylphosphoniumtetrafluoroborate; di(1-adamantyl)-N-butylphosphine hydroiodide In water; N,N-dimethyl-formamide; toluene at 80℃; for 16h; Suzuki-Miyaura Coupling; Inert atmosphere; Glovebox; | 83% |


| Conditions | Yield |
|---|---|
| With 2,6-di-tert-butyl-pyridine; bis(η3-allyl-μ-chloropalladium(II)); silver(I) triflimide In 1,2-dichloro-ethane at 65℃; under 22801.5 Torr; for 24h; Reagent/catalyst; Inert atmosphere; Sealed tube; Glovebox; Schlenk technique; | 83% |


- 61001-99-8
4-((4-chlorophenyl)(hydroxy)methyl)phenol


- 49562-28-9
fenofibrate

| Conditions | Yield |
|---|---|
| With oxygen; Langlois reagent In acetonitrile at 25℃; under 760.051 Torr; for 12h; Irradiation; | 83% |
- 70374-39-9Lornoxicam
- 22348-32-92-Pyrrolidinemethanol,a,a-diphenyl-, (2R)-
- 103041-38-9Ethyl 3-(pyridin-2-ylamino)propanoate
- 41100-52-1Memantine hydrochloride
- 132335-44-5(S)-(-)-N,N-Dimethyl-3-hydroxy-3-(2-thienyl)propanamine
- 7235-40-7beta-Carotene
- 52-51-71,3-Propanediol,2-bromo-2-nitro-
- 132539-06-110H-Thieno[2,3-b][1,5]benzodiazepine, 2-methyl-4-(4-methyl-1-piperazinyl)-
- 104987-11-3Tacrolimus
- 141-53-7Sodium formate
- 8001-54-5Quaternary ammonium compounds, alkylbenzyldimethyl, chlorides
- 9003-39-8Povidone
- 10161-34-9Trenbolone acetate
- 402957-28-2Telaprevir
- 68-19-9Cyanocobalamin
History
Fenofibrate (CAS NO.49562-28-9) was discovered by Groupe Fournier SA, before it was acquired in 2005 by Solvay Pharmaceutical, a business unit owned by the Belgian corporation, Solvay S.A. Fenofibrate is sold under the brand name Tricor and Trilipix by Abbott Labs, Lipofen by Kowa Pharmaceuticals America Inc, Lofibra by Teva, Lipanthyl and Lipidil by Solvay Pharmaceutical and Fenocor-67 by Ordain Health Care Pvt Ltd.
Consensus Reports
The pharmaceutical form and the strength may change from one country to another, and from one brand to another. In the United States, Tricor was reformulated in 2005 and is available in tablets of 48 and 145 mg. This reformulation is controversial and is the subject of antitrust litigation by generic drug manufacturer Teva. Also available in the United States, Lofibra is available in 54 and 160 mg tablets, as well as 67, 134, and 200 mg micronized capsules. Generic equivalents of Lofibra capsules are currently available in all three strengths in the United States. In Europe, it is available in either coated tablet or capsule; the strength range includes 67, 145, 160 and 200 mg. The differences among strengths are a result of altered bioavailability (the fraction absorbed by the body) due to particle size. For example, 200 mg can be replaced by 160 mg micronized fenofibrate. The 145 mg strength is a new strength appeared in 2005-2006 which also replaces 200 or 160 mg as the fenofibrate is nanonised (ie the particle size is below 400 nm).
Specification
Fenofibrate, with the IUPAC Name of propan-2-yl 2-[4-(4-chlorobenzoyl)phenoxy]-2-methylpropanoate, is one kind of drug. This chemical belongs to the Product Categories which include Active Pharmaceutical Ingredients; Intermediates & Fine Chemicals; Pharmaceuticals; Intracellular receptor. It is mainly used to reduce Cholesterol levels in patients at risk of cardiovascular disease. Like other fibrates, it reduces both low-density lipoprotein (LDL) and very low density lipoprotein (VLDL) levels, as well as increasing high-density lipoprotein (HDL) levels and reducing tryglycerides level. It also appears to have a beneficial effect on the Insulin resistance featured by the metabolic syndrome. It is used alone or in conjunction with statins in the treatment of hyperCholesterolemia and hypertriglyceridemia.
Physical properties about Fenofibrate are: (1)ACD/LogP: 5.801; (2)# of Rule of 5 Violations: 1; (3)ACD/LogD (pH 5.5): 5.80; (4)ACD/LogD (pH 7.4): 5.80; (5)ACD/BCF (pH 5.5): 15087.66; (6)ACD/BCF (pH 7.4): 15087.66; (7)ACD/KOC (pH 5.5): 34091.39; (8)ACD/KOC (pH 7.4): 34091.39; (9)#H bond acceptors: 4; (10)#Freely Rotating Bonds: 7; (11)Index of Refraction: 1.547; (12)Molar Refractivity: 97.116 cm3; (13)Molar Volume: 306.449 cm3; (14)Polarizability: 38.5 10-24cm3; (15)Surface Tension: 40.9819984436035 dyne/cm; (16)Density: 1.177 g/cm3; (17)Flash Point: 165.356 °C; (18)Enthalpy of Vaporization: 73.22 kJ/mol; (19)Boiling Point: 469.774 °C at 760 mmHg; (20)Vapour Pressure: 0 mmHg at 25°C
When you are using Fenofibrate, please be cautious about it as the following:
1.Wear suitable protective clothing;
2. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice;
You could convert the following datas into the molecular structure:
(1)InChI=1S/C20H21ClO4/c1-13(2)24-19(23)20(3,4)25-17-11-7-15(8-12-17)18(22)14-5-9-16(21)10-6-14/h5-13H,1-4H3;
(2)InChIKey=YMTINGFKWWXKFG-UHFFFAOYSA-N;
(3)Smilesc1(C(c2ccc(Cl)cc2)=O)ccc(OC(C(OC(C)C)=O)(C)C)cc1
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| dog | LD50 | oral | > 4gm/kg (4000mg/kg) | Yakuri to Chiryo. Pharmacology and Therapeutics. Vol. 23(Suppl, | |
| hamster | LD50 | oral | > 5gm/kg (5000mg/kg) | American Journal of Medicine. Vol. 83(Suppl, | |
| mouse | LD50 | oral | 1600mg/kg (1600mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 26, Pg. 885, 1976. | |
| rat | LD50 | oral | > 2gm/kg (2000mg/kg) | LIVER: OTHER CHANGES | Yakuri to Chiryo. Pharmacology and Therapeutics. Vol. 23(Suppl, |