Products Categories
| CAS No.: | 89-61-2 |
|---|---|
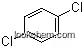
| Name: | 2,5-Dichloronitrobenzene |
| Article Data: | 51 |
| Molecular Structure: | |
|
|
|
| Formula: | C6H3Cl2NO2 |
| Molecular Weight: | 192.001 |
| Synonyms: | 2,5-Dichloronitrobenzol;2,5-Dichloro-1-nitrobenzene;Benzene, 2,5-dichloronitro-;Benzene, 1,4-dichloro-2-nitro-;2,5-Dichlornitrobenzen [Czech];2, 5-Dichlornitrobenzen;1-Nitro-2,5-dichlorobenzene;Nitro-p-dichlorobenzene;1,4-dichloro-2-nitro-benzene;1,4-Dichloro-2-nitrobenzene; |
| EINECS: | 201-923-3 |
| Density: | 1.533 g/cm3 |
| Melting Point: | 52-54 °C(lit.) |
| Boiling Point: | 267 °C at 760 mmHg |
| Flash Point: | 109.4 °C |
| Solubility: | Soluble in water, ethanol, ether, benzene, carbon disulfide. Slightly soluble in carbon tetrachloride. |
| Appearance: | yellow flakes |
| Hazard Symbols: |
 Xn; Xn;  N N
|
| Risk Codes: | 22-36-51/53 |
| Safety: | 26-60 |
| Transport Information: | UN 3077 9/PG 3 |
| PSA: | 45.82000 |
| LogP: | 3.42480 |
- 958254-66-51H-Imidazo[4,5-b]pyridine-2-carboxaldehyde, 1-methyl-, hydrochloride
- 99170-93-1N-Methyl-2-oxazolamine
- 914458-26-7[5-(2-fluorophenyl)-1-pentyl-1H-pyrrol-3-yl]-1-naphthalenyl-Methanone
- 90221-55-92-bromo-5-methylbenzaldehyde
- 97730-31-9(S)-4'-(2-Methylbutyl)Biphenyl-4-Carbonitrile
- 926293-55-26-Bromo-2-methylpyridine-3-carboxaldehyde
- 911112-05-55-Iodo-3-(trifluoromethyl)-2-pyridinamine
- 95789-13-21,3,7-trimethylpurine-2,6-dione
- 956104-42-02-Bromo-5-nitro-3-(trifluoromethyl)pyridine
- 910247-97-11,2,3-Trimethylimidazolium dimethyl phosphate

| Conditions | Yield |
|---|---|
| With fluoro alcohol In water; ethyl acetate; acetonitrile at -20℃; for 0.0833333h; | 97% |

| Conditions | Yield |
|---|---|
| With ortho-difluorobenzene; sulfuric acid; nitric acid at 35℃; Temperature; | 96.7% |
| With sulfuric acid; nitric acid at 0 - 23℃; for 0.283333h; Inert atmosphere; | 93% |
| With nitric acid |

| Conditions | Yield |
|---|---|
| With copper(l) iodide; oxygen; copper(l) chloride In dimethyl sulfoxide at 160℃; under 760.051 Torr; for 30h; Schlenk technique; Sealed tube; | 78% |
| With 2.9-dimethyl-1,10-phenanthroline; oxygen; copper (I) acetate; silver sulfate; sodium chloride In dimethyl sulfoxide at 160℃; for 24h; Schlenk technique; | 54% |
| With 2.9-dimethyl-1,10-phenanthroline; oxygen; copper diacetate; silver sulfate; sodium chloride In dimethyl sulfoxide at 160℃; under 760.051 Torr; for 20h; Schlenk technique; | 54% |
- 27165-22-6
2-nitro-4-chlorobenzenediazonium cation

A
- 56978-50-8
4,4'-dichloro-2,2'-dinitrobiphenyl

B
- 89-61-2
2,5-dichloronitrobenzene

| Conditions | Yield |
|---|---|
| With hydrogenchloride; copper dichloride |
- 10203-04-0
3,6-dichloro-2-nitro-benzaldehyde

- 89-61-2
2,5-dichloronitrobenzene

| Conditions | Yield |
|---|---|
| With potassium hydroxide at 100℃; |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; acetic acid at 40℃; anschliessendes Behandeln mit Kupfer(I)-chlorid und konz. wss. Salzsaeure bei 70grad; |

| Conditions | Yield |
|---|---|
| With antimonypentachloride beim Chlorieren; |

| Conditions | Yield |
|---|---|
| With antimonypentachloride bei der Chlorierung; |
- 121-73-3
3-Chloronitrobenzene

A
- 99-54-7
3,4-dichloronitrobenzene

B
- 3209-22-1
1,2-Dichloro-3-nitrobenzene

C
- 89-61-2
2,5-dichloronitrobenzene

| Conditions | Yield |
|---|---|
| With chlorine; iron(III) chloride at 90℃; Product distribution; relative rates; |

- 98-95-3
nitrobenzene

A
- 99-54-7
3,4-dichloronitrobenzene

B
- 121-73-3
3-Chloronitrobenzene

C
- 3209-22-1
1,2-Dichloro-3-nitrobenzene

D
- 100-00-5
4-chlorobenzonitrile

E
- 88-73-3
2-Chloronitrobenzene

F
- 89-61-2
2,5-dichloronitrobenzene

| Conditions | Yield |
|---|---|
| With chlorine; iron(III) chloride at 60 - 120℃; Product distribution; Kinetics; relative rates of each steps; |

- 109-92-2Ethyl vinyl ether
- 61-82-5Triazol-3-amine
- 93-07-2Benzoic acid,3,4-dimethoxy-
- 10191-41-0dl-alpha-Tocopherol
- 60-27-54H-Imidazol-4-one,2-amino-1,5-dihydro-1-methyl-
- 93793-83-0Roxatidine acetate hydrochloride
- 2768-02-7Vinyltrimethoxysilane
- 91-56-51H-Indole-2,3-dione
- 104987-11-3Tacrolimus
- 141-53-7Sodium formate
- 8001-54-5Quaternary ammonium compounds, alkylbenzyldimethyl, chlorides
- 9003-39-8Povidone
- 10161-34-9Trenbolone acetate
- 402957-28-2Telaprevir
- 68-19-9Cyanocobalamin
- Total:27 Page 1 of 1 1

What can I do for you?
Get Best Price
Consensus Reports
Specification
The 1,4-Dichloro-2-nitrobenzene, with the CAS registry number 89-61-2 and EINECS registry number 201-923-3, is also called 2,5-Dichloronitrobenzene. It is a kind of yellow flakes, and belongs to the following proudct categories: Intermediates of Dyes and Pigments; Aromatic Halides (substituted); Benzene derivates. And the molecular formula of this chemical is C6H3Cl2NO2. What's more, it is used as a kind of dye intermediate, and it is also used as nitrogen adjuvant.
The physical properties of 1,4-Dichloro-2-nitrobenzene are as following: (1)ACD/LogP: 2.95; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 2.95; (4)ACD/LogD (pH 7.4): 2.95; (5)#H bond acceptors: 3; (6)#H bond donors: 0; (7)#Freely Rotating Bonds: 1; (8)Polar Surface Area: 45.82 Å2; (9)Index of Refraction: 1.595; (10)Molar Refractivity: 42.58 cm3; (11)Molar Volume: 125.1 cm3; (12)Polarizability: 16.88×10-24cm3; (13)Surface Tension: 50.9 dyne/cm; (14)Density: 1.533 g/cm3; (15)Flash Point: 109.4 °C; (16)Enthalpy of Vaporization: 48.47 kJ/mol; (17)Boiling Point: 267 °C at 760 mmHg; (18)Vapour Pressure: 0.0138 mmHg at 25°C.
Preparation of 1,4-Dichloro-2-nitrobenzene: It can be prepared by p-dichlorobenzene via nitration, water scrubbing, neutralization and separation.

You should be cautious while dealing with this chemical. It irritates eyes, and it is also harmful if swallowed. What's more, it is very toxic to aquatic organisms, and may cause long-term adverse effects in the aquatic environment. Therefore, you had better take the following instructions: This material and/or its container must be disposed of as hazardous waste, and in case of contacting with eyes, rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
(1)SMILES: O=[N+]([O-])c1cc(Cl)ccc1Cl
(2)InChI: InChI=1/C6H3Cl2NO2/c7-4-1-2-5(8)6(3-4)9(10)11/h1-3H
(3)InChIKey: RZKKOBGFCAHLCZ-UHFFFAOYAY
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| guinea pig | LD50 | oral | 800mg/kg (800mg/kg) | National Technical Information Service. Vol. OTS0536149, | |
| mouse | LD50 | oral | 2850mg/kg (2850mg/kg) | Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 25(8), Pg. 50, 1981. | |
| rat | LD50 | oral | 1gm/kg (1000mg/kg) | Zhonghua Yufangyixue Zazhi. Chinese Journal of Preventive Medicine. Vol. 21, Pg. 315, 1987. |