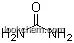Products Categories
| CAS No.: | 7722-76-1 |
|---|---|
| Name: | Ammonium dihydrogen orthophosphate |
| Molecular Structure: | |
|
|
|
| Formula: | NH6PO4 |
| Molecular Weight: | 115.026 |
| Synonyms: | azanium,dihydrogen phosphate;Ammonium dihydrogen phosphate; |
| EINECS: | 231-764-5 |
| Density: | 1.02 g/mL at 20 °C |
| Melting Point: | 190 °C (dec.)(lit.) |
| Boiling Point: | 158 °C at 760 mmHg |
| Solubility: | H2O: 0.1 M at 20 °C |
| Appearance: | white crystalline powder |
| Hazard Symbols: |
 Xi Xi
|
| Risk Codes: | 36/37/38 |
| Safety: | 24/25-36-26 |
| Transport Information: | UN 3264 |
| PSA: | 90.40000 |
| LogP: | -0.11420 |
- 81281-59-67-Benzylideneaminotheophylline
- 82993-81-5D-threo-Ritalinic acid hydrochloride
- 852475-26-4MC1568
- 958254-66-51H-Imidazo[4,5-b]pyridine-2-carboxaldehyde, 1-methyl-, hydrochloride
- 99170-93-1N-Methyl-2-oxazolamine
- 914458-26-7[5-(2-fluorophenyl)-1-pentyl-1H-pyrrol-3-yl]-1-naphthalenyl-Methanone
- 894852-01-87-BROMO-2,2-DIMETHYL-2H-PYRIDO[3,2-B][1,4]OXAZIN-3(4H)-ONE
- 90221-55-92-bromo-5-methylbenzaldehyde
- 885590-99-82,3-DIFLUORO-4-IODOBENZALDEHYDE
- 97730-31-9(S)-4'-(2-Methylbutyl)Biphenyl-4-Carbonitrile

- 86119-84-8, 7664-38-2
phosphoric acid


- 57-13-6
urea

A

- 7722-76-1
ammonium dihydrogen phosphate

B

- 124-38-9
carbon dioxide

| Conditions | Yield |
|---|---|
| With water Kinetics; |


| Conditions | Yield |
|---|---|
| With ammonium sulfate; sulfuric acid In not given byproducts: CaSO4; in presence of 16.2-22.2% (NH4)2SO4; | |
| With H2SO4; (NH4)2SO4 In not given byproducts: CaSO4; in presence of 16.2-22.2% (NH4)2SO4; |


- 86119-84-8, 7664-38-2
phosphoric acid


- 7722-76-1
ammonium dihydrogen phosphate

| Conditions | Yield |
|---|---|
| In not given with H3PO4 soln., at higher temp.; | |
| In not given with H3PO4 soln., at higher temp.; |



- 7722-76-1
ammonium dihydrogen phosphate

| Conditions | Yield |
|---|---|
| With ammonia; water at high temp., in sealed quarz tube in N2 flow; | |
| With NH3; H2O at high temp., in sealed quarz tube in N2 flow; |

- 14700-20-0, 2817-45-0
phosphoramidic acid


- 7722-76-1
ammonium dihydrogen phosphate

| Conditions | Yield |
|---|---|
| With phosphoramidic acid In water boiling; | |
| With phosphoramidic acid In water boiling; | |
| With water In water-d2 Kinetics; hydrolysis in D2O; catalyst: acids;; |

B

- 7722-76-1
ammonium dihydrogen phosphate

C

- 13446-12-3
ammonium dihydrogenphosphite

| Conditions | Yield |
|---|---|
| With water In neat (no solvent) heating in sealed tube at 215°C, 12 h; | |
| With H2O In neat (no solvent) heating in sealed tube at 215°C, 12 h; |


B

- 7722-76-1
ammonium dihydrogen phosphate

C

- 13446-12-3
ammonium dihydrogenphosphite

| Conditions | Yield |
|---|---|
| With water hydrolysis; | |
| With H2O hydrolysis; |


| Conditions | Yield |
|---|---|
| With water In neat (no solvent) heating in sealed tube at 215°C, 12 h; | |
| With H2O In neat (no solvent) heating in sealed tube at 215°C, 12 h; |

| Conditions | Yield |
|---|---|
| In not given at higher temp.; | |
| In not given at higher temp.; |

- 13446-12-3
ammonium dihydrogenphosphite


- 7722-76-1
ammonium dihydrogen phosphate

| Conditions | Yield |
|---|---|
| With water byproducts: H2; heated under pressure; | |
| With H2O byproducts: H2; heated under pressure; |
- 29908-03-0S-Adenosyl-L-methionine
- 111-40-01,2-Ethanediamine,N1-(2-aminoethyl)-
- 68439-46-3Alcohols,C9-11, ethoxylated
- 475207-59-1Sorafenib tosylate
- 106-89-8Epichlorohydrin
- 61791-12-6Cremophor EL
- 146725-34-08-Azabicyclo[3.2.1]octane-2-carboxylicacid, 3-(3,4-dichlorophenyl)-, methyl ester, (1R,2S,3S,5S)-
- 128-37-0Butylated hydroxytoluene
- 104987-11-3Tacrolimus
- 141-53-7Sodium formate
- 8001-54-5Quaternary ammonium compounds, alkylbenzyldimethyl, chlorides
- 9003-39-8Povidone
- 10161-34-9Trenbolone acetate
- 402957-28-2Telaprevir
- 68-19-9Cyanocobalamin

What can I do for you?
Get Best Price
Specification
Ammonium dihydrogen phosphate is also known as Monoammonium phosphate. It is a white tetragonal crystals with chemical formula NH4H2PO4. Its EINECS registry number is 231-764-5 and its CAS number is 7722-76-1. What's more, the substance is soluble in water which will melt at 190 °C. Ammonium dihydrogen phosphate can be mixed with all water-soluble fertilisers, except for calcium fertilisers and concentrated magnesium solutions. It should be kept in a tightly closed container, stored in a cool, dry, ventilated area and protected against physical damage. Ammonium dihydrogen phosphate is stable under ordinary conditions of use and storage.
Physical properties about Ammonium dihydrogen phosphate are: (1)ACD/LogP: -2.148; (2)ACD/LogD (pH 5.5): -5.41; (3)ACD/LogD (pH 7.4): -6.38; (4)ACD/BCF (pH 5.5): 1.00; (5)ACD/BCF (pH 7.4): 1.00; (6)#H bond acceptors: 4; (7)#H bond donors: 3; (8)Boiling Point: 158 °C at 760 mmHg; (9)Vapour Pressure: 1.41 mmHg at 25°C
Preparation of Ammonium dihydrogen phosphate: Ammonium dihydrogen phosphate can be prepared by venturi cyclic reaction method. Firstly, you should dilute 85% thermal phosphoric acid to 1.3~1.4 times and then add into reactor where it mixed and reacted with ammonia.
H3PO4 + NH3 → NH4H2PO4
There also are neutralization method and multiple decomposite method in industrial production, but neutralization method is more commonly used. It will need phosphoric acid and aqueous ammonia.
H3PO4 + NH4OH → NH4H2PO4 + H2O
Uses of Ammonium dihydrogen phosphate: Ammonium dihydrogen phosphate is often used in the blending of dry agricultural fertilizers. It is a widely used crystal in the field of optics due to its birefringence properties. The compound is also a component of the ABC powder in some dry chemical fire extinguishers. This substance is also supplied in an emerald green or aquamarine crystal growing box kit for kids.
When using Ammonium dihydrogen phosphate, you should very cautious about it. This chemical may cause inflammation to the skin or other mucous membranes. It is irritating to eyes, respiratory system and skin. In case of contact with eyes, you should rinse immediately with plenty of water and seek medical advice. Whenever you will contact it, please wear suitable protective clothing
You can still convert the following datas into molecular structure:
(1)Canonical SMILES: [NH4+].OP(=O)(O)[O-]
(2)InChI: InChI=1S/H3N.H3O4P/c;1-5(2,3)4/h1H3;(H3,1,2,3,4)
(3)InChIKey: LFVGISIMTYGQHF-UHFFFAOYSA-N