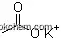Products Categories
| CAS No.: | 7440-09-7 |
|---|---|
| Name: | Potassium |
| Molecular Structure: | |
|
|
|
| Formula: | K |
| Molecular Weight: | 39.0983 |
| Synonyms: | Potassium(1+);Losartan Base;Kalium;Potassium, (liquid alloy);Potassium, metal; |
| EINECS: | 231-119-8 |
| Density: | 0.862 g/cm3 |
| Melting Point: | 63.38 °C |
| Boiling Point: | 759 °C |
| Solubility: | reacts with water violently |
| Appearance: | soft silvery metal |
| Hazard Symbols: |
 F, F, C C
|
| Risk Codes: | 14/15-34 |
| Safety: | 8-43-45-5B-5 |
| Transport Information: | UN 2257/1420 |
| PSA: | 0.00000 |
| LogP: | 0.11250 |
- 81281-59-67-Benzylideneaminotheophylline
- 82993-81-5D-threo-Ritalinic acid hydrochloride
- 852475-26-4MC1568
- 958254-66-51H-Imidazo[4,5-b]pyridine-2-carboxaldehyde, 1-methyl-, hydrochloride
- 99170-93-1N-Methyl-2-oxazolamine
- 914458-26-7[5-(2-fluorophenyl)-1-pentyl-1H-pyrrol-3-yl]-1-naphthalenyl-Methanone
- 894852-01-87-BROMO-2,2-DIMETHYL-2H-PYRIDO[3,2-B][1,4]OXAZIN-3(4H)-ONE
- 90221-55-92-bromo-5-methylbenzaldehyde
- 885590-99-82,3-DIFLUORO-4-IODOBENZALDEHYDE
- 97730-31-9(S)-4'-(2-Methylbutyl)Biphenyl-4-Carbonitrile


- 7440-09-7
potassium

| Conditions | Yield |
|---|---|
| With pyrographite In neat (no solvent) reduction of a small amount KOH starts at 700°C (in vac.) and proceeds at 900 °C;; | 90% |
| With pyrographite In neat (no solvent) reduction of a small amount KOH starts at 700°C (in vac.) and proceeds at 900 °C;; | 90% |
| With pyrographite In neat (no solvent) KOH is reduced by C at lower temp. than by Fe;; |


- 7440-09-7
potassium

| Conditions | Yield |
|---|---|
| With zirconium In neat (no solvent) K2WO4 + Zr (1:4), start of react.: 570 °C, calm react. process, yield: about 80% K, no oxide;; | 80% |
| With zirconium In neat (no solvent) pressed powder mixture (K2WO4:Zr = 1:4), heating in vac. to 570°C; mostly explosive reaction, with great excess of Zr smoothy;; further products;; | |
| With Zr In neat (no solvent) pressed powder mixture (K2WO4:Zr = 1:4), heating in vac. to 570°C; mostly explosive reaction, with great excess of Zr smoothy;; further products;; |

| Conditions | Yield |
|---|---|
| With zirconium In neat (no solvent) react. of pressed powd. K2CrO4/Zr mixt. (weight ratio 1:2 and 1:4) at 800 °C;; | A 0% B 80% |

- 20762-60-1
potassium azide


- 7440-09-7
potassium

| Conditions | Yield |
|---|---|
| In neat (no solvent) byproducts: N; heating 10-12g KN3 (fine powder) to 355°C (high vacuum, electrically heated tube from instrument glass (Jena)), decompn. starts sometimes 3-4h after obtaining temp., synthesis lasts some d;; spectroscopically pure and gas-free K obtained;; | 80% |
| In neat (no solvent) byproducts: N; thermal decompn. of KN3 (above 350°C, formation of N);; | |
| In neat (no solvent) byproducts: N; thermal decompn. of KN3 begins at 320°C and proceeds continually at 360°;; |


- 7440-09-7
potassium

| Conditions | Yield |
|---|---|
| With iron In neat (no solvent) byproducts: Fe2O3, FeS; heating react. mixture in a special apparatus for 2 h at 1000 °C, 1mm Hg; using of rough Fe-splints possible; further by-products: SO2 and O2;; | 80% |
| With iron In neat (no solvent) reductn. of K2SO4 by Fe in a quartz pot at 875 up to 1300 °C;; impured with 10 % sulfide;; | 60% |
| With zirconium In neat (no solvent) byproducts: K-oxide, K-sulfide; K2SO4 + Zr (1:4), start of react.: 725 °C, explosion, yield: 20% K, 20% K-oxide, 20% K-sulfide;; | 20% |

| Conditions | Yield |
|---|---|
| redn. with Zr powder in vac., 570°C; | 80% |
| redn. with Zr powder in vac., 570°C; | 80% |


- 7440-09-7
potassium

| Conditions | Yield |
|---|---|
| With zirconium In neat (no solvent) heating of K2MoO4 and Zr powder (1:4) in vac. at 500°C;; | 70% |
| With zirconium In neat (no solvent) K2MoO4 + Zr (1:4), start of react.: about 500 °C, calm react. process, yield: about 70% K, no oxide;; | 70% |
| With Zr In neat (no solvent) heating of K2MoO4 and Zr powder (1:4) in vac. at 500°C;; | 70% |
| With zirconium In neat (no solvent) pressed powder mixture (K2MoO4:Zr = 1:4), heating in vac. to about 500°C; mostly explosive reaction, with great excess of Zr smoothy;; further products;; | |
| With Zr In neat (no solvent) pressed powder mixture (K2MoO4:Zr = 1:4), heating in vac. to about 500°C; mostly explosive reaction, with great excess of Zr smoothy;; further products;; |


- 7440-09-7
potassium

| Conditions | Yield |
|---|---|
| With iron In neat (no solvent) byproducts: Fe-oxide, Fe-arsenide; heating of react. mixture in a special apparatus at 900 °C, 1mm Hg for 8h;; very pure and As-free K obtained;; | 50% |


- 7440-09-7
potassium

| Conditions | Yield |
|---|---|
| With zirconium In neat (no solvent) byproducts: K-oxide; K2Cr2O7 + Zr (1:4), start of react.: 370 °C, explosion, yield: 40% K, 30% oxide;; | 40% |
| With zirconium In neat (no solvent) pressed powder mixture (K2Cr2O7:Zr = 1:10), heating in vac. to 380°C; mostly explosive reaction, with great excess of Zr smoothy;; further products;; | |
| With aluminium In neat (no solvent) on ignition of a mixture of K2Cr2O7 and Al; vaporization of K;; |

| Conditions | Yield |
|---|---|
| With iron In neat (no solvent) byproducts: Fe2O3, CO, CO2; heating react. mixture in a special apparatus for 2 h at about 1000 °C, 1mm Hg; sucking off CO, CO2 (formation of explosive compounds possible);; | >99 |
| With aluminium In neat (no solvent) byproducts: Al2O3, C; heating of K2CO3 with Al in a stream of H2 to red heat; no potassium carbonyl is formed;; | >99 |
| With pyrographite In neat (no solvent) byproducts: CO; reduction of K2CO3 by C; no formation of K2O as intermediate;; |
 This product is a nationally controlled contraband, and the Lookchem platform doesn't provide relevant sales information.
This product is a nationally controlled contraband, and the Lookchem platform doesn't provide relevant sales information.
Consensus Reports
Reported in EPA TSCA Inventory.
Standards and Recommendations
DOT Classification: 4.3; Label: Dangerous When Wet
Specification
The IUPAC name of Potassium is potassium. With the CAS registry number 7440-09-7, it is also named as Kalium. The product's categories are Inorganic & Organic Chemicals; Inorganics, and the other registry number is 31079-13-7. Besides, it is soft silvery metal, which should be stored in kerosene or liquid paraffin. It is stable, but spontaneously combustible through the generation and ignition of hydrogen. In addition, its molecular formula is K and molecular weight is 39.09.
The other characteristics of this product can be summarized as: (1)EINECS: 231-119-8; (2)Exact Mass: 38.963707; (3)MonoIsotopic Mass: 38.963707; (4)Topological Polar Surface Area: 0; (5)Heavy Atom Count: 1; (6)Density: 0.862 g/cm3; (7)Melting point: 63.38 °C; (8)Boiling point: 759 °C; (9)Vapor pressure: 0.09 mm Hg ( 260 °C); (10)Triple point: 63 °C.
Preparation of Potassium: this chemical can be prepared by the reaction of Potassium chloride with Sodium. The reaction will occur by heating to 830 °C.
![]()
Uses of Potassium: this chemical is important in neuron (brain and nerve) function, and in influencing osmotic balance between cells and the interstitial fluid, with their distribution mediated in all animals (but not in all plants) by the so-called Na+/K+-ATPase pump. It is also used to prevent muscle contraction and the sending of all nerve impulses in animals through action potentials. Moreover, Potassium ion is a nutrient necessary for human life and health. It is used as reducing agent in organic synthesis. Furthermore, it is widely used in conjunction with loop diuretics and thiazides, classes of diuretics which rid the body of sodium and water in medicine.
When you are using this chemical, please be cautious about it as the following: it reacts violently with water in contact with water liberates extremely flammable gases. Please keep contents under the oil. And you should keep container dry. It also may cause burns. In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
People can use the following data to convert to the molecule structure.
(1)SMILES: [K]
(2)InChI: InChI=1/K
(3)InChIKey: ZLMJMSJWJFRBEC-UHFFFAOYAX
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | 700mg/kg (700mg/kg) | "Structure et Activite Pharmacodyanmique des Medicaments du Systeme Nerveux Vegetatif," Bovet, D., and F. Bovet-Nitti, New York, S. Karger, 1948Vol. -, Pg. 704, 1948. |