Products Categories
| CAS No.: | 654671-77-9 |
|---|---|
| Name: | Sitagliptin phosphate monohydrate |
| Article Data: | 6 |
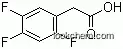
| Molecular Structure: | |
|
|
|
| Formula: | C16H15F6N5O.H3PO4.H2O |
| Molecular Weight: | 523.329 |
| Synonyms: | 7-[(3R)-3-Amino-1-oxo-4-(2,4,5-trifluorophenyl)butyl]-5,6,7,8-tetrahydro-3-(trifluoromethyl)-1,2,4-triazolo[4,3-a]pyrazine phosphate monohydrate;Sitagliptin phosphate; |
| EINECS: | 682-492-2 |
| Boiling Point: | 529.9 °C at 760 mmHg |
| Flash Point: | 274.3 °C |
| Appearance: | White or almost white crystalline powder |
| Risk Codes: | 28-38-41-48-62-63 |
| Safety: | 24/25-26-28-36/37/39 |
| PSA: | 173.84000 |
| LogP: | 1.66180 |
- 81281-59-67-Benzylideneaminotheophylline
- 82993-81-5D-threo-Ritalinic acid hydrochloride
- 73441-42-6METHYL-5-CHLORO-2,2-DIMETHYLVALERATE
- 68439-39-4Poly(oxy-1,2-ethanediyl), alpha-(2-ethylhexyl)-omega-hydroxy-,
- 852475-26-4MC1568
- 958254-66-51H-Imidazo[4,5-b]pyridine-2-carboxaldehyde, 1-methyl-, hydrochloride
- 99170-93-1N-Methyl-2-oxazolamine
- 717878-06-31-(4-fluorophenyl)-4-nitro-1H-imidazole
- 914458-26-7[5-(2-fluorophenyl)-1-pentyl-1H-pyrrol-3-yl]-1-naphthalenyl-Methanone
- 894852-01-87-BROMO-2,2-DIMETHYL-2H-PYRIDO[3,2-B][1,4]OXAZIN-3(4H)-ONE

- 486460-32-6
sitagliptin


- 654671-77-9
sitagliptin phosphate monohydrate

| Conditions | Yield |
|---|---|
| With phosphoric acid In water; isopropyl alcohol at 70 - 80℃; for 2h; Temperature; | 97.7% |
| With phosphoric acid; water In isopropyl alcohol at 20 - 75℃; | 95% |
| With phosphoric acid; water In isopropyl alcohol at 75℃; |

- 764667-65-4
1-(3-(trifluoromethyl)-5,6-dihydro-[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl)-4-(2,4,5-trifluorophenyl)butane-1,3-dione


- 654671-77-9
sitagliptin phosphate monohydrate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: isopropyl alcohol; acetic acid / 18 h / 50 °C 2: sodium tetrahydroborate; formic acid / tetrahydrofuran / -30 °C 3: 20% palladium hydroxide-activated charcoal; hydrogen; acetic acid / methanol; water / 14 h / 50 °C / 7500.75 Torr 4: phosphoric acid; water / isopropyl alcohol / 20 - 75 °C View Scheme | |
| Multi-step reaction with 4 steps 1: isopropyl alcohol; acetic acid / 18 h / 50 °C 2: sodium tetrahydroborate; acetic acid / tetrahydrofuran / 3 h / 20 °C 3: 20% palladium hydroxide-activated charcoal; hydrogen; acetic acid / methanol; water / 14 h / 50 °C / 7500.75 Torr 4: phosphoric acid; water / isopropyl alcohol / 20 - 75 °C View Scheme | |
| Multi-step reaction with 3 steps 1.1: ammonium acetate; ammonia / methanol; water / 2 h / 30 °C / Heating / reflux 2.1: (R, S) t-butyl Josiphos / chloro(1,5-cyclooctadiene)rhodium(I) dimer / methanol / 1 h / 20 °C 2.2: 13 h / 50 °C / 10343.2 Torr 3.1: phosphoric acid; water / isopropyl alcohol / 2 h / 68 - 75 °C View Scheme |

- 1169707-29-2
(R,Z)-3-[(1-phenylethyl)amino]-1-{3-(trifluoromethyl)-5,6-dihydro-[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl}-4-(2,4,5-trifluorophenyl)but-2-en-1-one


- 654671-77-9
sitagliptin phosphate monohydrate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: sodium tetrahydroborate; formic acid / tetrahydrofuran / -30 °C 2: 20% palladium hydroxide-activated charcoal; hydrogen; acetic acid / methanol; water / 14 h / 50 °C / 7500.75 Torr 3: phosphoric acid; water / isopropyl alcohol / 20 - 75 °C View Scheme | |
| Multi-step reaction with 3 steps 1: sodium tetrahydroborate; acetic acid / tetrahydrofuran / 3 h / 20 °C 2: 20% palladium hydroxide-activated charcoal; hydrogen; acetic acid / methanol; water / 14 h / 50 °C / 7500.75 Torr 3: phosphoric acid; water / isopropyl alcohol / 20 - 75 °C View Scheme |

- 1169707-30-5
(3R)-3-[[(1R)-1-phenylethyl]amino]-1-[3-(trifluoromethyl)-6,8-dihydro-5H-[1,2,4]triazolo[4,3-a]pyrazin-7-yl]-4-(2,4,5-trifluorophenyl)butan-1-one


- 654671-77-9
sitagliptin phosphate monohydrate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 20% palladium hydroxide-activated charcoal; hydrogen; acetic acid / methanol; water / 14 h / 50 °C / 7500.75 Torr 2: phosphoric acid; water / isopropyl alcohol / 20 - 75 °C View Scheme |

- 868071-17-4
3(S)-4-(2,4,5-trifluorophenyl)-3-hydroxybutanoic acid


- 654671-77-9
sitagliptin phosphate monohydrate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: lithium hydroxide; dicyclohexyl-carbodiimide / tetrahydrofuran; water; cis-1,2-Dichloroethylene / 3 h / 20 - 22 °C 1.2: 18.5 h / 10 - 20 °C 2.1: lithium hydroxide / tetrahydrofuran; water / 2.33 h / 20 - 25 °C / pH 3 2.2: 3 h / 0 - 5 °C 3.1: phosphoric acid / isopropyl alcohol / 16 h / 30 °C 4.1: sodium hydroxide / water / 1 h / 0 - 5 °C 5.1: phosphoric acid; water / water; isopropyl alcohol / 0.25 h View Scheme |

- 767352-30-7
N-(benzyloxy)-4(R)-[1-methyl-(2,4,5-trifluorophenyl)]-2-oxoazetidine


- 654671-77-9
sitagliptin phosphate monohydrate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: lithium hydroxide / tetrahydrofuran; water / 2.33 h / 20 - 25 °C / pH 3 1.2: 3 h / 0 - 5 °C 2.1: phosphoric acid / isopropyl alcohol / 16 h / 30 °C 3.1: sodium hydroxide / water / 1 h / 0 - 5 °C 4.1: phosphoric acid; water / water; isopropyl alcohol / 0.25 h View Scheme |

- 1361389-75-4
3(R)-3-[(benzyloxy)amino]-1-[3-(trifluoromethyl)-5H,6H,7H,8H-[1,2,4]triazolo[4,3-a]pyrazin-7-yl]-4-(2,4,5-trifluorophenyl)butan-1-one


- 654671-77-9
sitagliptin phosphate monohydrate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: phosphoric acid / isopropyl alcohol / 16 h / 30 °C 2: sodium hydroxide / water / 1 h / 0 - 5 °C 3: phosphoric acid; water / water; isopropyl alcohol / 0.25 h View Scheme |


- 654671-77-9
sitagliptin phosphate monohydrate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sodium hydroxide / water / 1 h / 0 - 5 °C 2: phosphoric acid; water / water; isopropyl alcohol / 0.25 h View Scheme |

- 769195-26-8
4-(2,4,5-trifluoro-phenyl)-3-oxo-butyric acid methyl ester


- 654671-77-9
sitagliptin phosphate monohydrate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1.1: hydrogen; dichloro[(S)-(-)-2,2'-bis(diphenylphosphino)-1,1'-binaphthyl]ruthenium(II) / methanol; acetic acid / 70 °C / 3620.13 Torr / Inert atmosphere; Autoclave 2.1: lithium hydroxide; dicyclohexyl-carbodiimide / tetrahydrofuran; water; cis-1,2-Dichloroethylene / 3 h / 20 - 22 °C 2.2: 18.5 h / 10 - 20 °C 3.1: lithium hydroxide / tetrahydrofuran; water / 2.33 h / 20 - 25 °C / pH 3 3.2: 3 h / 0 - 5 °C 4.1: phosphoric acid / isopropyl alcohol / 16 h / 30 °C 5.1: sodium hydroxide / water / 1 h / 0 - 5 °C 6.1: phosphoric acid; water / water; isopropyl alcohol / 0.25 h View Scheme |

- 209995-38-0
(2,4,5-trifluorophenyl)acetic acid


- 654671-77-9
sitagliptin phosphate monohydrate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1.1: triethylamine; magnesium chloride / acetonitrile / 30 - 50 °C / Inert atmosphere 1.2: 5.5 h / 30 °C / Inert atmosphere 2.1: hydrogen; dichloro[(S)-(-)-2,2'-bis(diphenylphosphino)-1,1'-binaphthyl]ruthenium(II) / methanol; acetic acid / 70 °C / 3620.13 Torr / Inert atmosphere; Autoclave 3.1: lithium hydroxide; dicyclohexyl-carbodiimide / tetrahydrofuran; water; cis-1,2-Dichloroethylene / 3 h / 20 - 22 °C 3.2: 18.5 h / 10 - 20 °C 4.1: lithium hydroxide / tetrahydrofuran; water / 2.33 h / 20 - 25 °C / pH 3 4.2: 3 h / 0 - 5 °C 5.1: phosphoric acid / isopropyl alcohol / 16 h / 30 °C 6.1: sodium hydroxide / water / 1 h / 0 - 5 °C 7.1: phosphoric acid; water / water; isopropyl alcohol / 0.25 h View Scheme | |
| Multi-step reaction with 5 steps 1.1: dmap; N-ethyl-N,N-diisopropylamine / N,N-dimethyl acetamide / 20 - 40 °C 1.2: 2 - 3 h / 0 - 5 °C 2.1: N,N-dimethyl acetamide / 1 h / 40 - 70 °C 2.2: 3 - 5 h / 20 - 45 °C 3.1: ammonium acetate; ammonia / methanol; water / 2 h / 30 °C / Heating / reflux 4.1: (R, S) t-butyl Josiphos / chloro(1,5-cyclooctadiene)rhodium(I) dimer / methanol / 1 h / 20 °C 4.2: 13 h / 50 °C / 10343.2 Torr 5.1: phosphoric acid; water / isopropyl alcohol / 2 h / 68 - 75 °C View Scheme |
- 274901-16-52-Pyrrolidinecarbonitrile,1-[2-[(3-hydroxytricyclo[3.3.1.13,7]dec-1-yl)amino]acetyl]-, (2S)-
- 102767-28-2Levetiracetam
- 137234-62-9Voriconazole
- 73-22-3L-Tryptophan
- 202138-50-9Tenofovir disoproxil fumarate
- 8013-07-8Soybean oil, epoxidized
- 360-70-3Nandrolone decanoate
- 98319-26-7Finasteride
- 104987-11-3Tacrolimus
- 141-53-7Sodium formate
- 8001-54-5Quaternary ammonium compounds, alkylbenzyldimethyl, chlorides
- 9003-39-8Povidone
- 10161-34-9Trenbolone acetate
- 402957-28-2Telaprevir
- 68-19-9Cyanocobalamin

What can I do for you?
Get Best Price
Chemistry
IUPAC name: (3R)-3-Amino-1-[3-(trifluoromethyl)-6,8-dihydro-5H-[1,2,4]triazolo[4,3-a]pyrazin-7-yl]-4-(2,4,5-trifluorophenyl)butan-1-one ; phosphoric acid ; hydrate
Synonyms: 7-((3R)-3-Amino-4-(2,4,5-trifluorophenyl)butanoyl)-3-(trifluoromethyl)-5,6,7,8-tetrahydro-1,2,4- triazolo(4,3-
a)pyrazine monophosphate monohydrate ; Sitagliptin phosphate hydrate
Molecular Formula: C16H20F6N5O6P
Molecular Weight: 523.32408 g/mol
Flash Point: 274.3 °C
Enthalpy of Vaporization: 80.51 kJ/mol
Boiling Point: 529.9 °C at 760 mmHg
Vapour Pressure: 2.59E-11 mmHg at 25 °C
Following is the Structure of Sitagliptin phosphate monohydrate (CAS NO.654671-77-9):
.png)
History
Sitagliptin phosphate monohydrate (CAS NO.654671-77-9) was approved by the U.S. Food and Drug Administration (FDA) on October 17, 2006 and is marketed in the US as Januvia by Merck & Co. On April 2, 2007, the FDA approved an oral combination of sitagliptin and metformin marketed in the US as Janumet.
Uses
1、Sitagliptin phosphate monohydrate (CAS NO.654671-77-9) works to competitively inhibit the enzyme dipeptidyl peptidase 4 (DPP-4). This enzyme breaks down the incretins GLP-1 and GIP, gastrointestinal hormones that are released in response to a meal.
2、Sitagliptin phosphate monohydrate also has an effect on appetite. By slowing down gastric motility it induces a feeling of satiety. This reduction of appetite can help patients to lose weight, a useful effect in patients with diabetes.
Specification
In clinical trials, adverse effects were as common with sitagliptin (whether used alone or with metformin or pioglitazone) as they were with placebo, except for extremely rare nausea and common cold-like symptoms.There is no significant difference in the occurrence of hypoglycemia between placebo and sitagliptin .