Products Categories
| CAS No.: | 56-86-0 |
|---|---|
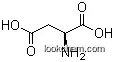
| Name: | L-Glutamic acid (alpha) |
| Article Data: | 429 |
| Molecular Structure: | |
|
|
|
| Formula: | C5H9NO4 |
| Molecular Weight: | 147.131 |
| Synonyms: | 1-Aminopropane-1,3-dicarboxylic acid;2-Aminopentanedioic acid;2-Aminopentanedioic acid, (S)-;AI3-18472;Acide glutamique;Acido glutamico;Acidum glutamicum;Acidum glutaminicum;Aciglut;CCRIS 7314;D-Glutamiensuur;EPA Pesticide Chemical Code 374350;FEMA No. 3285;Glusate;Glutacid;Glutamate, L-;Glutamic acid (H-3);Glutamic acid (VAN);Glutamic acid, (S)-;Glutamicol;Glutamidex;Glutaminic acid;Glutaminic acid (VAN);Glutaminol;Glutaton;L-2-Aminoglutaric acid;L-Glutaminic acid;NSC 143503;Pentanedioic acid, 2-amino-, (S)-;UNII-3KX376GY7L;alpha-Aminoglutaric acid;alpha-Aminoglutaric acid (VAN); |
| EINECS: | 200-293-7 |
| Density: | 1.41 g/cm3 |
| Melting Point: | 205 °C (dec.)(lit.) |
| Boiling Point: | 333.783 °C at 760 mmHg |
| Flash Point: | 155.667 °C |
| Solubility: | 7.5 g/L (20 °C) in water |
| Appearance: | White cryst. powder |
| Hazard Symbols: |
 Xi Xi
|
| Risk Codes: | 36/37/38 |
| Safety: | 24/25-36-26 |
| PSA: | 100.62000 |
| LogP: | -0.03660 |
- 81281-59-67-Benzylideneaminotheophylline
- 82993-81-5D-threo-Ritalinic acid hydrochloride
- 73441-42-6METHYL-5-CHLORO-2,2-DIMETHYLVALERATE
- 68439-39-4Poly(oxy-1,2-ethanediyl), alpha-(2-ethylhexyl)-omega-hydroxy-,
- 852475-26-4MC1568
- 958254-66-51H-Imidazo[4,5-b]pyridine-2-carboxaldehyde, 1-methyl-, hydrochloride
- 99170-93-1N-Methyl-2-oxazolamine
- 717878-06-31-(4-fluorophenyl)-4-nitro-1H-imidazole
- 914458-26-7[5-(2-fluorophenyl)-1-pentyl-1H-pyrrol-3-yl]-1-naphthalenyl-Methanone
- 894852-01-87-BROMO-2,2-DIMETHYL-2H-PYRIDO[3,2-B][1,4]OXAZIN-3(4H)-ONE


- 56-86-0
L-glutamic acid

| Conditions | Yield |
|---|---|
| With dimethylsulfide; hydrogen fluoride; methoxybenzene at 0℃; for 1h; Product distribution; Rate constant; other concentration of reagents; | 100% |
| With dimethylsulfide; trifluorormethanesulfonic acid; 30 (v/v); trifluoroacetic acid at 0℃; for 4h; Yield given; | |
| With trifluoroacetic acid In dichloromethane at 20℃; Rate constant; | |
| With recombinant Pseudomonas nitroreducens IFO12694 γ-glutamyltranspeptidase at 30℃; for 0.0333333h; pH=10.5; aq. buffer; Enzymatic reaction; | |
| With E. coli BL21 Star (DE3) S30 extract In aq. buffer at 37℃; for 6h; pH=7.5; |

- 13574-13-5
Boc-Glu(OBzl)-OH


- 56-86-0
L-glutamic acid

| Conditions | Yield |
|---|---|
| With Nafion H; dimethylsulfide; 3-methyl-phenol; trifluoroacetic acid for 3h; | 100% |
| With dimethylsulfide; hydrogen fluoride at 0℃; for 2h; Product distribution; Var.: HF in anisole; | 100% |

- 23506-06-1
Z(OMe)-Glu(OBzl)-OH


- 56-86-0
L-glutamic acid

| Conditions | Yield |
|---|---|
| With trimethylsilyl trifluoromethanesulfonate; diphenyl sulfide In trifluoroacetic acid at 0℃; for 0.5h; Product distribution; New peptide deprotection procedure: hard-soft acid-base concept; the role of soft bases (thioanisole, dimethylsulfide, diphenylsulfide) employed.; | 100% |

- 73821-97-3
N-α-tert-butyloxycarbonyl-glutamic acid, γ-cyclohexyl ester


- 56-86-0
L-glutamic acid

| Conditions | Yield |
|---|---|
| With phenylthiotrimethylsilane; pertrimethylsilylated Nafion; 3-methyl-phenol; trifluoroacetic acid for 3h; | 100% |

- 91871-28-2
N-allyloxycarbonyl α-allyl-L-glutamate


- 56-86-0
L-glutamic acid

| Conditions | Yield |
|---|---|
| With diethylamine; tetrakis(triphenylphosphine) palladium(0) In dichloromethane at 20℃; for 3h; deallylation; | 100% |

| Conditions | Yield |
|---|---|
| With hydrogen In ethanol for 1.33333h; | 98% |
| With hydrogen; hydroxyapatite-bound Pd In methanol at 40℃; for 12h; | 96% |
| With hydrogen In ethanol at 20℃; under 760.051 Torr; for 3h; | 95% |

- 132316-96-2
(S)-(N-benzyl-N-benzyloxycarbonyl)glutamic acid


- 56-86-0
L-glutamic acid

| Conditions | Yield |
|---|---|
| With hydrogen; palladium dihydroxide In water; acetic acid for 45h; Ambient temperature; | 97% |

- 13726-84-6
Boc-Glu(OtBu)-OH


- 56-86-0
L-glutamic acid

| Conditions | Yield |
|---|---|
| With triethylsilane; trifluoroacetic acid In dichloromethane for 1.08333h; Ambient temperature; | 96% |

| Conditions | Yield |
|---|---|
| With tetradecyl(trihexyl)phosphonium bistriflamide; trifluoroacetic acid at 130℃; for 0.166667h; Ionic liquid; | 96% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; zinc(II) sulfate In water at 105℃; for 50h; Product distribution; Mechanism; Heating; at pH 6.5; variation of amount of substrate and added salts, reaction time and temperature; further metal salts; | 93.1% |
| Rate constant; | |
| Multi-step reaction with 7 steps 1.1: 100 percent / 1,3-dicyclohexylcarbodiimide; 4-(dimethylamino)pyridine / CH2Cl2 / 0 - 20 °C 2.1: 100 percent / 3-chloroperoxybenzoic acid / CH2Cl2 / 4 h / 0 °C 3.1: bis(cyclopentadienyl)zirconium dichloride; silver(I) perchlorate / CH2Cl2 / 48 h 4.1: 9.40 g / 4-(dimethylamino)pyridine / acetonitrile / 1.5 h / 20 °C 5.1: sodium bis(trimethylsilyl)amide; N,N'-dimethylpropyleneurea / tetrahydrofuran / 0.5 h / -78 °C 5.2: tetrahydrofuran / 2 h 6.1: 6.48 g / hydrogen peroxide / tetrahydrofuran / 2 h / 20 °C 7.1: hydrogen / palladium on carbon / methanol 7.2: 77 percent / hydrochloric acid / Heating View Scheme |
- 10605-21-7Carbendazim
- 1115-70-4Metformin hydrochloride
- 107534-96-3Tebuconazole
- 372-75-8L(+)-Citrulline
- 7758-16-9Disodium pyrophosphate
- 129722-12-9Aripiprazole
- 99300-78-4Venlafaxine hydrochloride
- 112529-15-4Pioglitazone hydrochloride
- 104987-11-3Tacrolimus
- 141-53-7Sodium formate
- 8001-54-5Quaternary ammonium compounds, alkylbenzyldimethyl, chlorides
- 9003-39-8Povidone
- 10161-34-9Trenbolone acetate
- 402957-28-2Telaprevir
- 68-19-9Cyanocobalamin

What can I do for you?
Get Best Price
Consensus Reports
Reported in EPA TSCA Inventory.
Specification
1. Introduction of L-Glutamic acid
L-Glutamic acid, with the IUPAC Name of (2S)-2-Aminopentanedioic acid, is one kind of white cryst. powder. This chemical belongs to the Product Categories which include Food & Feed ADDITIVES; chiral; Glutamic acid [Glu, E]; Amino Acids and Derivatives; alpha-Amino Acids; Amino Acids; Biochemistry; for Resolution of Bases; Optical Resolution; Synthetic Organic Chemistry; Nutritional Supplements; Amino Acids; Chiral Compound; Glutamate receptor; Glutamate; fine chemicals; glutamic acid; amino acid; bio-chemical; chemicals; food additive; food additives; nutritional supplement; organic acids; pharmaceutical intermediate.
2. Properties of L-Glutamic acid
L-Glutamic acid has the following property datas: (1)Melting point: 205 °C; (2)Index of Refraction: 1.522; (3)Molar Refractivity: 31.83 cm3; (4)Molar Volume: 104.3 cm3; (5)Surface Tension: 69.2 dyne/cm; (6)Density: 1.409 g/cm3; (7)Flash Point: 155.7 °C; (8)Enthalpy of Vaporization: 63.39 kJ/mol; (9)Boiling Point: 333.8 °C at 760 mmHg; (10)Vapour Pressure: 2.55E-05 mmHg at 25 °C; (11)storage temp.: Store at RT.; (12)solubility: 1 M HCl: 100 mg/mL; (13)Water Solubility: 7.5 g/L (20 °C).
3. Structure Descriptors of L-Glutamic acid
You could convert the following datas into the molecular structure:
InChI: InChI=1S/C5H9NO4/c6-3(5(9)10)1-2-4(7)8/h3H,1-2,6H2,(H,7,8)(H,9,10)/t3-/m0/s1
InChIKey: InChIKey=WHUUTDBJXJRKMK-VKHMYHEASA-N
Smiles: [C@H](CCC(=O)O)(C(=O)O)N
4. Toxicity of L-Glutamic acid
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| human | TDLo | intravenous | 117mg/kg (117mg/kg) | GASTROINTESTINAL: NAUSEA OR VOMITING | American Journal of the Medical Sciences. Vol. 214, Pg. 281, 1947. |
| human | TDLo | oral | 71mg/kg (71mg/kg) | BEHAVIORAL: HEADACHE | Science. Vol. 163, Pg. 826, 1969. |
| rabbit | LD50 | oral | > 2300mg/kg (2300mg/kg) | FAO Nutrition Meetings Report Series. Vol. 48A, Pg. 42, 1970. | |
| rat | LD50 | oral | > 30gm/kg (30000mg/kg) | FAO Nutrition Meetings Report Series. Vol. 48A, Pg. 42, 1970. |
5. Safety Information of L-Glutamic acid
Hazard Codes of L-Glutamic acid (CAS NO.56-86-0):
 Xi
Xi Risk Statements: 36/37/38
R36/37/38: Irritating to eyes, respiratory system and skin.
Safety Statements: 24/25-36-26
S24/25: Avoid contact with skin and eyes.
S36: Wear suitable protective clothing.
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
WGK Germany: 2
RTECS: LZ9700000
F: 10
Human systemic effects by ingestion and intravenous routes: headache and nausea or vomiting. When heated to decomposition it emits toxic fumes of NOx.
6. Production of L-Glutamic acid
L-Glutamic acid can be obtained directly from fermentation of carbohydrates with Micrococcus glutarnicus or Brevihacterium divaricatum.
-
Premium Related Products