Products Categories
| CAS No.: | 123447-62-1 |
|---|---|
| Name: | Prulifloxacin |
| Article Data: | 9 |
| Molecular Structure: | |
|
|
|
| Formula: | C21H20FN3O6S |
| Molecular Weight: | 461.471 |
| Synonyms: | 1H,4H-[1,3]Thiazeto[3,2-a]quinoline-3-carboxylic acid,6-fluoro-1-methyl-7-[4-[(5-methyl- 2-oxo-1,3-dioxol-4-yl)methyl]-1- piperazinyl]-4-oxo-;PUFX;(+-)-7-(4-((Z)-2,3-Dihydroxy-2-butenyl)-1-piperazinyl)-6-fluoro-1-methyl-4-oxo-1H,4H-(1,3)thiazeto(3,2-a)quinoline-3-carboxylic acid, cyclic carbonate;Sword;Sword (TN); |
| EINECS: | 819-932-1 |
| Density: | 1.62 g/cm3 |
| Melting Point: | 211-214°C |
| Boiling Point: | 633.2 °C at 760 mmHg |
| Flash Point: | 336.8 °C |
| Solubility: | Slightly soluble in dimethyl formamide, 0.1M NaOH or glacial acetic acid; insoluble in water or methanol |
| Appearance: | Yellow or slightly yellow power |
| PSA: | 134.43000 |
| LogP: | 2.64270 |
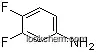
- 144851-82-1METHYL2-AMINO-3-FLUOROBENZOATE
- 483366-12-7(2S,4R)-1-Boc-2-cyano-4-hydroxypyrrolidine
- 173606-50-3BOC-10-AMINODECANOIC ACID
- 361456-36-2METHYL (R)-(+)-ISOCYANATO-3-PHENYLPROPI&
- 5156-58-1N-(1-Benzyl-4-pipperidinyl)-N-phenylpropanamide HCl
- 81281-59-67-Benzylideneaminotheophylline
- 50288-62-5threo-Phenyl-2-piperidyl acetamide
- 82993-81-5D-threo-Ritalinic acid hydrochloride
- 47087-37-6Z-D-Glu-OMe
- 73441-42-6METHYL-5-CHLORO-2,2-DIMETHYLVALERATE

- 80715-22-6
4-bromomethyl-1,3-dioxa-5-methylcyclopentene-2-one


- 112984-60-8
ulifloxacin


- 123447-62-1
prulifloxacin

| Conditions | Yield |
|---|---|
| With potassium hydrogencarbonate In N,N-dimethyl-formamide at 0℃; for 5h; Large scale; | 95.4% |
| With potassium hydrogencarbonate In N,N-dimethyl-formamide for 5h; Ambient temperature; | 62% |
| Stage #1: ulifloxacin With potassium hydrogencarbonate In acetonitrile at 25 - 30℃; Stage #2: 4-bromomethyl-1,3-dioxa-5-methylcyclopentene-2-one at 15 - 30℃; for 27 - 28.25h; | |
| Stage #1: 4-bromomethyl-1,3-dioxa-5-methylcyclopentene-2-one; ulifloxacin With potassium hydrogencarbonate In N,N-dimethyl-formamide at 0 - 28℃; for 3 - 4h; Stage #2: With hydrogenchloride In chloroform; water for 0.25h; pH=0.8 - 1.0; | |
| Stage #1: ulifloxacin With N-ethyl-N,N-diisopropylamine In acetonitrile at 20℃; for 0.166667h; Stage #2: 4-bromomethyl-1,3-dioxa-5-methylcyclopentene-2-one In acetonitrile at 10 - 30℃; for 21h; Product distribution / selectivity; |

- 80715-22-6
4-bromomethyl-1,3-dioxa-5-methylcyclopentene-2-one


- 112984-60-8
ulifloxacin

A

- 123447-62-1
prulifloxacin

B

- 156834-57-0
6-fluoro-1-methyl-4-oxo-7-<4-<(2-oxo-1-methylenepropyl)oxycarbonyl>-1-piperazinyl>-4H-<1,3>thiazeto<3,2-a>quinoline-3-carboxylic acid


| Conditions | Yield |
|---|---|
| With potassium hydrogencarbonate In N,N-dimethyl-formamide at 30℃; for 2h; Product distribution; Rate constant; various periods of time (3 h); activation energy 29.1 kJ mol-1; | A 93.6 % Spectr. B 2.0 % Spectr. C 0.5 % Spectr. D 1.1 % Spectr. |


- 80715-22-6
4-bromomethyl-1,3-dioxa-5-methylcyclopentene-2-one


- 112984-60-8
ulifloxacin

A

- 123447-62-1
prulifloxacin

| Conditions | Yield |
|---|---|
| With potassium hydrogencarbonate for 3h; Product distribution; also in presence of DIPEA, car. concn. of substrates; |


| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine; sodium bromide 1.) DMF, 303 K, 2 h, 2.) DMF, 304 K, 1 h; Yield given. Multistep reaction; |

- 113028-75-4
3,4-difluorophenyl isothiocyanate


- 123447-62-1
prulifloxacin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: 1.) KOH / 1.) dioxane, RT, 30 min., 2.) 4 deg C, 15 h 2: 2.22 g / K2CO3 / dimethylformamide / 1 h / 70 °C 3: 0.350 g / PPE / 1.5 h / 80 °C 4: 84 percent / dimethylformamide / Ambient temperature 5: 88 percent / KOH / 2-methyl-propan-2-ol; H2O / 1 h / 50 - 60 °C 6: 62 percent / KHCO3 / dimethylformamide / 5 h / Ambient temperature View Scheme | |
| Multi-step reaction with 8 steps 1: 1.) KOH / 1.) dioxane, RT, 30 min., 2.) 4 deg C, 15 h 2: dimethylformamide / 1.) 0 deg C, 1.5 h, 2.) RT, 2 h 3: 58 percent / diphenyl ether / 5 h / 240 °C 4: 99 percent / 35percent HCl / ethanol / 2 h / Ambient temperature 5: 47 percent / K2CO3, KI / dimethylformamide / 0.5 h / 105 - 110 °C 6: 84 percent / dimethylformamide / Ambient temperature 7: 88 percent / KOH / 2-methyl-propan-2-ol; H2O / 1 h / 50 - 60 °C 8: 62 percent / KHCO3 / dimethylformamide / 5 h / Ambient temperature View Scheme |

- 84339-06-0
ethyl 7,6-difluoro-4-hydroxy-2-mercaptoquinoline-3-carboxylate


- 123447-62-1
prulifloxacin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 47 percent / K2CO3, KI / dimethylformamide / 0.5 h / 105 - 110 °C 2: 84 percent / dimethylformamide / Ambient temperature 3: 88 percent / KOH / 2-methyl-propan-2-ol; H2O / 1 h / 50 - 60 °C 4: 62 percent / KHCO3 / dimethylformamide / 5 h / Ambient temperature View Scheme |

- 144514-15-8
3,4-difluorophenyl dithiocarbamic acid triethylammonium


- 123447-62-1
prulifloxacin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: ethyl chloroformate, triethylamine / CHCl3 / 3.5 h / Ambient temperature 2: 1.) KOH / 1.) dioxane, RT, 30 min., 2.) 4 deg C, 15 h 3: 2.22 g / K2CO3 / dimethylformamide / 1 h / 70 °C 4: 0.350 g / PPE / 1.5 h / 80 °C 5: 84 percent / dimethylformamide / Ambient temperature 6: 88 percent / KOH / 2-methyl-propan-2-ol; H2O / 1 h / 50 - 60 °C 7: 62 percent / KHCO3 / dimethylformamide / 5 h / Ambient temperature View Scheme | |
| Multi-step reaction with 9 steps 1: ethyl chloroformate, triethylamine / CHCl3 / 3.5 h / Ambient temperature 2: 1.) KOH / 1.) dioxane, RT, 30 min., 2.) 4 deg C, 15 h 3: dimethylformamide / 1.) 0 deg C, 1.5 h, 2.) RT, 2 h 4: 58 percent / diphenyl ether / 5 h / 240 °C 5: 99 percent / 35percent HCl / ethanol / 2 h / Ambient temperature 6: 47 percent / K2CO3, KI / dimethylformamide / 0.5 h / 105 - 110 °C 7: 84 percent / dimethylformamide / Ambient temperature 8: 88 percent / KOH / 2-methyl-propan-2-ol; H2O / 1 h / 50 - 60 °C 9: 62 percent / KHCO3 / dimethylformamide / 5 h / Ambient temperature View Scheme |

- 113046-72-3
ethyl 6,7-difluoro-1-methyl-4-oxo-1H,4H-[1,3]thiazeto[3,2-a]quinoline-3-carboxylate


- 123447-62-1
prulifloxacin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 84 percent / dimethylformamide / Ambient temperature 2: 88 percent / KOH / 2-methyl-propan-2-ol; H2O / 1 h / 50 - 60 °C 3: 62 percent / KHCO3 / dimethylformamide / 5 h / Ambient temperature View Scheme |

- 113028-77-6
ethyl 6,7-difluoro-4-hydroxy-2-<(methoxymethyl)thio>-quinoline-3-carboxylate


- 123447-62-1
prulifloxacin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 99 percent / 35percent HCl / ethanol / 2 h / Ambient temperature 2: 47 percent / K2CO3, KI / dimethylformamide / 0.5 h / 105 - 110 °C 3: 84 percent / dimethylformamide / Ambient temperature 4: 88 percent / KOH / 2-methyl-propan-2-ol; H2O / 1 h / 50 - 60 °C 5: 62 percent / KHCO3 / dimethylformamide / 5 h / Ambient temperature View Scheme |

- 144514-35-2
3-(3,4-difluorophenyl)-4-methyl<1,3>thiazetidin-2-ylidenemalonate


- 123447-62-1
prulifloxacin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 0.350 g / PPE / 1.5 h / 80 °C 2: 84 percent / dimethylformamide / Ambient temperature 3: 88 percent / KOH / 2-methyl-propan-2-ol; H2O / 1 h / 50 - 60 °C 4: 62 percent / KHCO3 / dimethylformamide / 5 h / Ambient temperature View Scheme |
- 557795-19-4Sunitinib
- 109-64-81,3-Dibromopropane
- 617-86-7Silane,triethyl-
- 144-68-3Zeaxanthin
- 62-46-4Lipoic acid
- 89-65-6D-Isoascorbic acid
- 54827-17-7Tetramethylbenzidine
- 108-48-5Pyridine, 2,6-dimethyl-
- 104987-11-3Tacrolimus
- 141-53-7Sodium formate
- 8001-54-5Quaternary ammonium compounds, alkylbenzyldimethyl, chlorides
- 9003-39-8Povidone
- 10161-34-9Trenbolone acetate
- 402957-28-2Telaprevir
- 68-19-9Cyanocobalamin
Chemistry
Molecular Structure of Prulifloxacin (CAS NO.123447-62-1):
.png)
IUPAC Name: (1R)-6-fluoro-1-methyl-7-[4-[(5-methyl-2-oxo-1,3-dioxol-4-yl)methyl]piperazin-1-yl]-4-oxo-1H-[1,3]thiazeto[3,2-a]quinoline-3-carboxylic acid
CAS Registry Number 123447-62-1
Molecular Formula:C21H20FN3O6S
Molecular Weight:461.46
H bond acceptors: 9
H bond donors: 1
Freely Rotating Bonds: 4
Polar Surface Area: 113.92 Å2
Molar Volume: 283.5 cm3
Surface Tension: 82 dyne/cm
Index of Refraction: 1.72
Surface Tension: 82 dyne/cm
Density: 1.62 g/cm3
Flash Point: 336.8 °C
Enthalpy of Vaporization: 98.39 kJ/mol
Boiling Point: 633.2 °C at 760 mmHg
Vapour Pressure: 6.62E-17 mmHg at 25°C
Product Categories: API
Solubility: Slightly soluble in dimethyl formamide, 0.1M NaOH or glacial acetic acid; insoluble in water or methanol
Appearance: Yellow or slightly yellow power
InChI
InChI=1/C21H20FN3O6S/c1-10-16(31-21(29)30-10)9-23-3-5-24(6-4-23)15-8-14-12(7-13(15)22)18(26)17(20(27)28)19-25(14)11(2)32-19/h7-8,11H,3-6,9H2,1-2H3,(H,27,28)
Smiles
o1c(oc(CN2CCN(CC2)c2c(cc3c(c2)n2c(c(c3=O)C(O)=O)S[C@@H]2C)F)c1C)=O
Specification
Prulifloxacin , its cas register number is 123447-62-1. It also can be called (+/-)-7-{4-[(Z)-2,3-Dihydroxy-2-butenyl]-1-piperazinyl}-6-fluoro-1-methyl-4-oxo-1H,4H-[1,3]thiazeto[3,2-a]quinoline-3-carboxylic acid cyclic carbonate ; 6-Fluoro-1-methyl-7-(4-(5-methyl-2-oxo-1,3-dioxelen-4-yl)methyl-1-piperazinyl)-4-oxo-4H-(1,3)thiazeto(3,2-a)quinoline-3-carboxylic acid , it can be used as gatifloxacin for the whole synthetic antibacterial drugs.