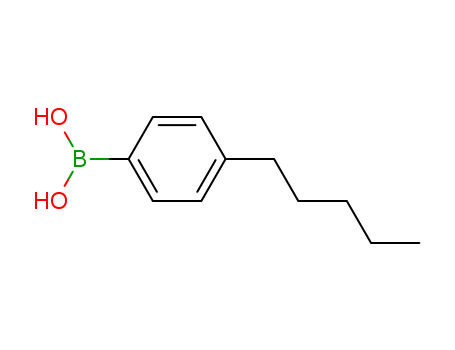
- Chemical Name:(4-pentylphenyl)boronic Acid
- CAS No.:121219-12-3
- Molecular Formula:C11H17BO2
- Molecular Weight:192.066
- Hs Code.:29319090
- European Community (EC) Number:681-163-0
- ChEMBL ID:CHEMBL1952296
- DSSTox Substance ID:DTXSID50404610
- Nikkaji Number:J1.102.040B
- Pharos Ligand ID:ZR7Y666HH5CF
- Wikidata:Q72500114
- Mol file:121219-12-3.mol
Synonyms:121219-12-3;(4-pentylphenyl)boronic Acid;4-n-Pentylphenylboronic acid;4-Pentylphenylboronic Acid;4-Pentylbenzeneboronic acid;4-N-PENTYLBENZENEBORONIC ACID;4-Amylbenzeneboronic Acid;MFCD00995151;CHEMBL1952296;Boronic acid, (4-pentylphenyl)-;4-Amylphenylboronic Acid;4-n-pentylphenyl boronic acid;AKOS BRN-0130;4-n-pentylbenzene boronic acid;SCHEMBL1509988;DTXSID50404610;UZRMPSOGFATLJE-UHFFFAOYSA-N;BCP22757;BDBM50364283;AKOS004116473;AB07922;BCP9000264;CS-W002722;GS-6717;4-N-Pentylphenylboronic acid, AldrichCPR;AC-24820;SY015715;AM20050555;FT-0619317;A804694;J-515894;(4-pentylphenyl)boronic acid


 Xi
Xi


