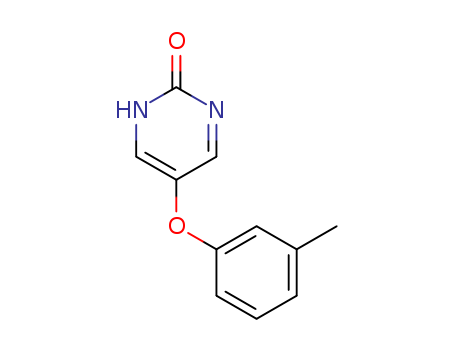
- Chemical Name:Tolimidone
- CAS No.:41964-07-2
- Molecular Formula:C11H10N2O2
- Molecular Weight:202.213
- Hs Code.:2933599090
- European Community (EC) Number:803-991-5
- NSC Number:314335
- UNII:MU3JD8E9IS
- DSSTox Substance ID:DTXSID50194786
- Nikkaji Number:J16.876I
- Wikipedia:Tolimidone
- Wikidata:Q7814266
- NCI Thesaurus Code:C152683
- Pharos Ligand ID:HGQ51BAPVYBU
- Metabolomics Workbench ID:144596
- ChEMBL ID:CHEMBL8030
- Mol file:41964-07-2.mol
Synonyms:5-(3-methylphenoxy)-2(1H)-pyrimidinone;MLR-1023