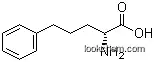
2046-19-7Relevant articles and documents
Effective synthesis of optically active 3-phenyl-3-(3-trifluoromethyl) diazirinyl bishomophenylalanine derivatives
Murai, Yuta,Hatanaka, Yasumaru,Kanaoka, Yuichi,Hashimoto, Makoto
, p. 359 - 364 (2009)
Effective incorporation of phenyldiazirine moiety on the acyl residue of L- and D- glutamic acid by Friedel-Crafts reactions with triflic acid developed simple preparation of bishomophenylalanine (bhPhe) for aromatics, which added a
Synthesis method of multi-configuration long-chain phenyl amino acid compound
-
, (2021/03/05)
The invention relates to a method for synthesizing a multi-configuration long-chain phenyl amino acid compound. The method comprises the following steps: reacting a compound shown in a formula I witha compound shown in a formula II or an isomer thereof in
Controlling diastereoselectivity in the reactions of enantiomerically pure α-bromoacyl-imidazolidinones with nitrogen nucleophiles: Substitution reactions with retention or inversion of configuration
Treweeke,Hitchcock,Pardoe,Caddick
, p. 1868 - 1870 (2007/10/03)
Diastereoselective substitution reactions of α-bromoacyl- imidazolidinones with nitrogen nucleophiles can be promoted with either retention or inversion of configuration by carrying out reactions under epimerising or non-epimerising conditions. The Royal Society of Chemistry 2005.
Inhibitors of cathepsin S
-
Page 23, (2010/02/08)
The present invention provides compounds, compositions and methods for the selective inhibition of cathepsin S. In a preferred aspect, cathepsin S is selectively inhibited in the presence of at least one other cathepsin isozyme (e.g., cathespin K). The pr
STEREOSELECTIVE PROCESS FOR THE SYNTHESIS OF (D)-2-AMINO-5-PHENYLPENTANOIC OR ALKYL ESTER THEREOF
-
Page/Page column 9-10, (2010/02/07)
The present invention provides a process for preparing a compound of formula (I); wherein R is a C1-C6 alkyl; or a salt thereof; comprising:(c) hydrolyzing (D,L)-N-acetyl-2-amino-5-phenylpentanoic acid with a suitable base in the pre
Treatment of insulin resistance with growth hormone secretagogues
-
, (2008/06/13)
This invention is directed to methods of treating insulin resistance in a mammal which comprise administering an effective amount of a compound of formula I, where the variables are defined in the specification, or the stereoisomeric mixtures, diastereomerically enriched, diastereomerically pure, enantiomerically enriched or enantiomerically pure isomers, or the pharmaceutically acceptable salts and prodrugs thereof to said mammal. The compounds of formula I are growth hormone secretagogues and as such are useful for increasing the level of endogenous growth hormone. In another aspect this invention provides certain intermediates which are useful in the synthesis of the foregoing compounds and certain processes useful for the synthesis of said intermediates and the compounds of formula I. This invention is further directed to methods comprising administering to a human or other animal a combination of a functional somatostatin antagonist such as an alpha-2 adrenergic agonist and a compound of formula I.
Tricyclic erythromycin derivatives
-
, (2008/06/13)
Compounds, or pharmaceutically acceptable salts and esters thereof, of the formula: wherein A, B, D and E, R1, R2, and Z are specifically defined, having antibacterial activity, pharmaceutical compositions containing said compounds, treatment of bacterial infections with such compositions, and processes for the preparation of the compounds.
Heterocyclic compounds
-
, (2008/06/13)
This invention is directed to compounds of the formula and the pharmaceutically-acceptable salts thereof, where the substituents are as defined in the Specification, which are growth hormone secretogogues and which increase the level of endogenous growth hormone. The compounds of this invention are useful for the treatment and prevention of osteoporosis, congestive heart failure, frailty associated with aging, obesity; accelerating bone fracture repair, attenuating protein catabolic response after a major operation, reducing cachexia and protein loss due to chronic illness, accelerating wound healing, or accelerating the recovery of burn patients or patients having undergone major surgery; improving muscle strength, mobility, maintanence of skin thickness, metabolic homeostasis or renal homeostasis. The compounds of the present invention are also useful in treating osteoporosis when used in combination with: a bisphosphonate compound such as alendronate; estrogen, premarin, and optionally progesterone; an estrogen agonist or antagonist; or calcitonin, and pharmaceutical compositions useful therefor. Further, the present invention is directed to pharmaceutical compositions useful for increasing the endogenous production or release of growth hormone in a human or other animal which comprises an effective amount of a compound of the present invention and a growth hormone secretagogue selected from GHRP-6, Hexarelin, GHRP-1, growth hormone releasing factor (GRF), IGF-1, IGF-2 or B-HT920. The invention is also directed to intermediates useful in the preparation of compounds of formula I.
SULFONAMIDE INHIBITORS OF MATRIX METALLOPROTEINASES
-
, (2008/06/13)
Sulfonamide compounds are described which are inhibitors of matrix metalloproteinases, particularly stromelysin-1 and gelatinase A (72 kD gelatinase). Also described are methods for the treatment of multiple sclerosis, atherosclerotic plaque rupture, aortic aneurism, heart failure, restenosis, periodontal disease, corneal ulceration, burns, decubital ulcers, chronic ulcers or wounds, cancer metastasis, tumor angiogenesis, arthritis, or other autoimmune or inflammatory disorders dependent upon tissue invasion by leukocytes using the compounds.