Products Categories
| CAS No.: | 14977-61-8 | |
|---|---|---|
| Name: | Chromyl chloride | |
| Article Data: | 142 | |
| Molecular Structure: | ||
|
|
||
| Formula: | Cl2Cr O2 | |
| Molecular Weight: | 154.901 | |
| Synonyms: | Chromium,dichlorodioxo- (8CI);Chromyl chloride (6CI);Chromium chloride oxide(CrCl2O2);Chromium chloride oxide (CrO2Cl2);Chromium dichloride dioxide;Chromium dioxide dichloride;Chromium oxide chloride (CrO2Cl2);Chromiumoxychloride (CrCl2O2);Chromium oxychloride (CrO2Cl2);Chromium(VI)dioxychloride;Chromyl chloride (CrCl2O2);Chromyl chloride (CrO2Cl2);Chromyldichloride;Dichlorodioxochromium; | |
| EINECS: | 239-056-8 | |
| Density: | 1.911 g/mL at 25 °C(lit.) | |
| Melting Point: | -96.5 °C | |
| Boiling Point: |
117 °C(lit.) |
|
| Flash Point: | 117°C | |
| Solubility: | Soluble in carbon tetrachloride, carbon disulfide, benzene, nitrobenzene, chloroform, and POCL3. Insoluble in water(Reacts). | |
| Hazard Symbols: | TLV: 0.025 ppm. Corrosive to tissue. Strong oxidizing agent. | |
| Risk Codes: | R35;R43;R46;R49;R50/53;R8; | |
| Safety: | Suspected carcinogen. Probably a poison by various routes. Mutation data reported. Corrosive. A strong irritant. Hydrolyzes to form chromic and hydrochloric acids. A strong oxidizer and chlorinating agent. Violent reaction with water. Reacts violently with alcohol, ether, acetone, turpentine. Ignites or explodes on contact with nonmetal halides (e.g., disulfur dichloride, phosphorus trichloride, and phosphorus tribromide), nonmetal hydrides (e.g., hydrogen sulfide and hydrogen phosphide), flowers of sulfur, moist phosphorus, sodium azide, and urea. During preparation can violently explode. Incompatible with ammonia, disulfur dichloride, organic solvents, phosphorus, phosphorus trichloride, sodium azide, and sulfur. When heated to decomposition it emits toxic fumes of Cl−. See also CHROMIUM COMPOUNDS. | |
Analytical Methods: | For occupational chemical analysis use NIOSH: Chromium Hexavalent 7024. |
|
| PSA: | 34.14000 | |
| LogP: | 1.14140 | |
- 483366-12-7(2S,4R)-1-Boc-2-cyano-4-hydroxypyrrolidine
- 173606-50-3BOC-10-AMINODECANOIC ACID
- 361456-36-2METHYL (R)-(+)-ISOCYANATO-3-PHENYLPROPI&
- 5156-58-1N-(1-Benzyl-4-pipperidinyl)-N-phenylpropanamide HCl
- 81281-59-67-Benzylideneaminotheophylline
- 50288-62-5threo-Phenyl-2-piperidyl acetamide
- 82993-81-5D-threo-Ritalinic acid hydrochloride
- 47087-37-6Z-D-Glu-OMe
- 73441-42-6METHYL-5-CHLORO-2,2-DIMETHYLVALERATE
- 211914-50-0N-[[2-[[[4-(Aminoiminomethyl)phenyl]amino]methyl]-1-methyl-1H-benzimidazol-5-yl]carbonyl]-N-(2-pyridinyl)-beta-alanine ethyl ester hydrochloride
- Total:8 Page 1 of 1 1
Chemistry
Molecular Structure of Chromyl chloride (CAS NO. 14977-61-8):
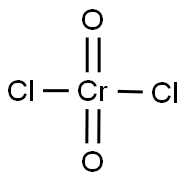
Name: Chromyl chloride
EINECS: 239-056-8
Molecular Formula: Cl2CrO2
Molecular Weight: 154.9
Density: 1.911 g/mL at 25 °C(lit.)
Melting Point: -96.5 °C
Flash Point: 117°C
Boiling Point: 117 °C(lit.)
Sensitive: Moisture
Toxicity Data With Reference
| 1. | mmo-sat 50 µg/plate | CRNGDP Carcinogenesis. 1 (1980),583. | ||
| 2. | mma-sat 100 µg/plate | CRNGDP Carcinogenesis. 1 (1980),583. |
Consensus Reports
Reported in EPA TSCA Inventory. Chromium and its compounds are on the Community Right-To-Know List.
Safety Profile
Safety Information of Chromyl chloride (CAS NO. 14977-61-8):
Hazard Codes: O  ,T
,T  ,C
,C  ,N
,N 
Risk Statements: 49-46-8-35-43-50/53-34-23/24/25-45
R49:May cause cancer by inhalation
R46:May cause heritable genetic damage
R8 :Contact with combustible material may cause fire
R35:Causes severe burns
R50/53:Very toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment
R34:Causes burns
R23/24/25:Toxic by inhalation, in contact with skin and if swallowed
R45:May cause cancer
Safety Statements: 53-45-60-61-36/37/39-27-26-17
S53:Avoid exposure - obtain special instructions before use
S45:In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
S60:This material and its container must be disposed of as hazardous waste
S61:Avoid release to the environment. Refer to special instructions / safety data sheets
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection
S27:Take off immediately all contaminated clothing
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
S17:Keep away from combustible material
RIDADR: UN 3244 8/PG 2
WGK Germany: 3
RTECS: GB5775000
HazardClass: 8
PackingGroup: I
F: 8-10-21
Suspected carcinogen. Probably a poison by various routes. Mutation data reported. Corrosive. A strong irritant. Hydrolyzes to form chromic and hydrochloric acids. A strong oxidizer and chlorinating agent. Violent reaction with water. Reacts violently with alcohol, ether, acetone, turpentine. Ignites or explodes on contact with nonmetal halides (e.g., disulfur dichloride, phosphorus trichloride, and phosphorus tribromide), nonmetal hydrides (e.g., hydrogen sulfide and hydrogen phosphide), flowers of sulfur, moist phosphorus, sodium azide, and urea. During preparation can violently explode. Incompatible with ammonia, disulfur dichloride, organic solvents, phosphorus, phosphorus trichloride, sodium azide, and sulfur. When heated to decomposition it emits toxic fumes of Cl−. See also CHROMIUM COMPOUNDS.
Standards and Recommendations
OSHA PEL: TWA 0.5 mg(Cr)/m3
ACGIH TLV: TWA 0.025 ppm
DFG MAK: Suspected Carcinogen
NIOSH REL: (Chromium(VI)) TWA 0.001 mg(Cr(VI))/m3
DOT Classification: 8; Label: Corrosive
Analytical Methods
For occupational chemical analysis use NIOSH: Chromium Hexavalent 7024.
Specification
Chromyl chloride with cas registry number of 14977-61-8 is also known as Chlorochromic anhydride ; Chlorure de chromyle ; Chlorure de chromyle [French] ; Chromic oxychloride ; Chromium (VI) dioxychloride ; Chromium chloride oxide ; Chromium chloride oxide (CrO2Cl2) ; Chromium dichloride dioxide ; Chromium dioxide dichloride ; Chromium oxychloride (CrO2Cl2) ; Chromium(VI) dioxychloride ; Chromium, dichlorodioxo- ; Chromoxychlorid ; Chromoxychlorid [German] ; Chromyl chloride (CrO2Cl2) ; Chromylchlorid ; Chromylchlorid [German] ; Chroomoxychloride ; Chroomoxychloride [Dutch] ; Chromium oxychloride [UN1758] [Corrosive] ; Chromyl chloride [Chromium and chromium compounds] ; Cromile, cloruro di ; Cromile, cloruro di [Italian] ; Cromo, ossicloruro di ; Cromo, ossicloruro di [Italian] ; Chromium, dichlorodioxo-, (T-4)- ; Chromyl dichloride ; Dichlorodioxochromium ; Dioxodichlorochromium ; HSDB 990 ; Oxychlorure chromique ; Oxychlorure chromique [French] ; UN1758 . It is a dark red fuming liquid with a pungent odor. Chromyl chloride is a powerful and often violent oxidizing agent and reacts readily with many inorganic and organic materials in the absence of a dilutent. It can also used as solvent for chromic acid, chromium complex, dyes in organic synthesis. It can react with water to release hydrochloric acid and hexavalent chromium . It is incompatible with water , reducing agents, alcohols.

