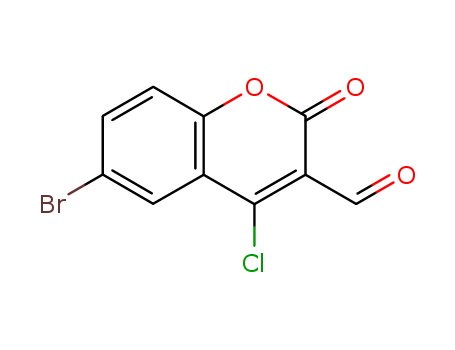
- Chemical Name:6-Bromo-4-chloro-3-formylcoumarin
- CAS No.:51069-90-0
- Molecular Formula:C10H4BrClO3
- Molecular Weight:287.49
- Hs Code.:2932209090
- European Community (EC) Number:624-094-3
- DSSTox Substance ID:DTXSID30405241
- Nikkaji Number:J996.053H
- Wikidata:Q82209694
- Mol file:51069-90-0.mol
Synonyms:6-Bromo-4-chloro-3-formylcoumarin;51069-90-0;6-bromo-4-chloro-2-oxo-2h-chromene-3-carbaldehyde;6-bromo-4-chloro-2-oxochromene-3-carbaldehyde;6-Bromo-4-chloro-3-formylcoumarin 97;starbld0000776;SCHEMBL9910631;DTXSID30405241;AKOS015916286;6-Bromo-4-chloro-3-formylcoumarin, 97%;CS-0119932;E81449;A899736;A937241


 Xi
Xi


