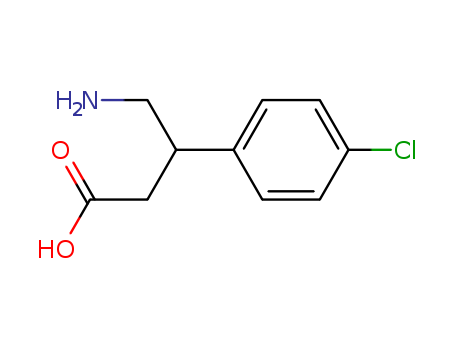
- Chemical Name:Baclofen
- CAS No.:1134-47-0
- Molecular Formula:C10H12ClNO2
- Molecular Weight:213.664
- Hs Code.:2922492050
- European Community (EC) Number:214-486-9
- NSC Number:755906,329137
- UN Number:2811
- UNII:H789N3FKE8
- DSSTox Substance ID:DTXSID5022641
- Nikkaji Number:J3.661G
- Wikipedia:Baclofen
- Wikidata:Q413717
- NCI Thesaurus Code:C28858
- RXCUI:1292
- Pharos Ligand ID:USJMSKZ6V3XF
- Metabolomics Workbench ID:42589
- ChEMBL ID:CHEMBL701
- Mol file:1134-47-0.mol
Synonyms:Apo Baclofen;Apo-Baclofen;ApoBaclofen;Atrofen;AWD, Baclofen;Ba-34,647;Ba-34647;Ba34,647;Ba34647;Baclofène Irex;Baclofène-Irex;BaclofèneIrex;Baclofen;Baclofen AWD;Baclophen;Baclospas;beta-(Aminomethyl)-4-chlorobenzenepropanoic Acid;beta-(p-Chlorophenyl)-gamma-aminobutyric Acid;Chlorophenyl GABA;CIBA-34,647-BA;CIBA34,647BA;Clofen;GABA, Chlorophenyl;Gen Baclofen;Gen-Baclofen;GenBaclofen;Genpharm;Lebic;Liorésal;Lioresal;Nu Baclo;Nu-Baclo;NuBaclo;PCP-GABA;PMS Baclofen;PMS-Baclofen;PMSBaclofen


 T,
T, Xn
Xn

