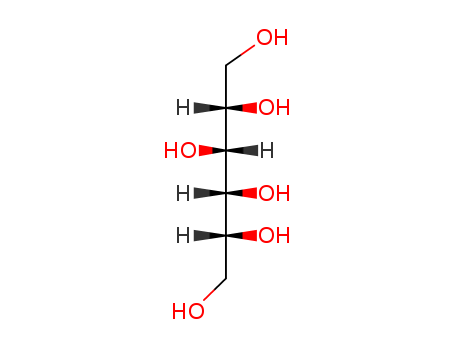
- Chemical Name:Sorbitol
- CAS No.:50-70-4
- Deprecated CAS:15060-73-8,36134-87-9,3959-53-3,63800-20-4,75398-79-7,8013-15-8,8014-89-9,8036-93-9,8042-39-5,8045-74-7,8046-05-7,98201-93-5,952319-42-5,36134-87-9,3959-53-3,63800-20-4,8013-15-8,8014-89-9,8036-93-9,8042-39-5,8045-74-7,8046-05-7,952319-42-5,98201-93-5
- Molecular Formula:C6H14O6
- Molecular Weight:182.174
- Hs Code.:2905.44 Oral rat LD50: 15900 mg/kg
- European Community (EC) Number:200-061-5
- ICSC Number:0892
- NSC Number:759608
- UNII:506T60A25R
- DSSTox Substance ID:DTXSID5023588
- Nikkaji Number:J2.299C
- Wikipedia:Sorbitol
- Wikidata:Q245280
- NCI Thesaurus Code:C29462
- RXCUI:9945
- Metabolomics Workbench ID:37159
- ChEMBL ID:CHEMBL1682
- Mol file:50-70-4.mol
Synonyms:Sorbitol syrup C;Sorbitur;Sorbogem 712;Sorbostyl;Sorbitol Powder;Sorbitol (7CI);(-)-Sorbitol;C*Sorbidex;C*Sorbidex P 16616;CSorbidex 16100;Cholaxine;Cystosol;D-Sorbit 50M;Glucarine;Glucitol;Karion;Karioninstant;L-Gulitol;Multitol;NSC 25944;Neosorb;Neosorb 20/60DC;Neosorb 70/02SB;Neosorb 70/70;Neosorb P 60;Nivitin;Resulax;Sionit K;Sionite;Siosan;Sorbex R;Sorbex Rp;Sorbex S;Sorbidex S 16601;Sorbit LTSP;Sorbit T 70;Sorbit W-Powder;Sorbit WP;Sorbitol F;Sorbitol FP;Sorbitol S;


 Xi
Xi


