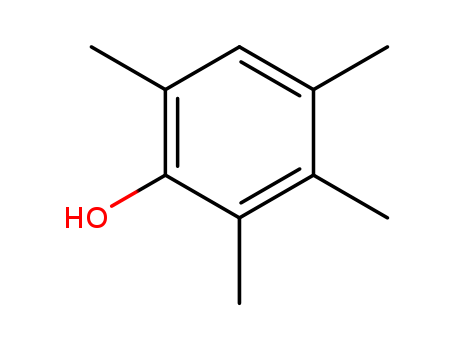
- Chemical Name:2,3,4,6-Tetramethylphenol
- CAS No.:3238-38-8
- Deprecated CAS:28449-98-1
- Molecular Formula:C10H14 O
- Molecular Weight:150.221
- Hs Code.:2907199090
- European Community (EC) Number:221-799-4
- UNII:O8267G3UF3
- DSSTox Substance ID:DTXSID5062926
- Nikkaji Number:J38.246I
- Wikidata:Q27285458
- ChEMBL ID:CHEMBL4287899
- Mol file:3238-38-8.mol
Synonyms:2,3,4,6-Tetramethylphenol;Phenol, 2,3,4,6-tetramethyl-;Isodurol;3238-38-8;isodurenol;UNII-O8267G3UF3;O8267G3UF3;EINECS 221-799-4;SCHEMBL394530;CHEMBL4287899;DTXSID5062926;AKOS006272177;EN300-7691564;Q27285458