- Chemical Name:Oxotremorine
- CAS No.:70-22-4
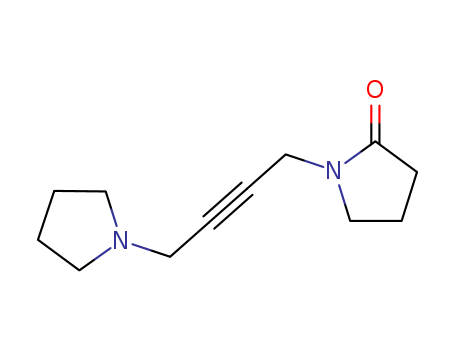
- Molecular Formula:C12H18N2O
- Molecular Weight:206.288
- Hs Code.:
- European Community (EC) Number:200-728-0
- NSC Number:330497
- UNII:5RY0UWH1JL
- DSSTox Substance ID:DTXSID10220252
- Nikkaji Number:J1.421D
- Wikipedia:Oxotremorine
- Wikidata:Q676896
- NCI Thesaurus Code:C67048
- Pharos Ligand ID:NUX1N7J2S33B
- Metabolomics Workbench ID:67481
- ChEMBL ID:CHEMBL7634
- Mol file:70-22-4.mol
Synonyms:Oxotremorine;Oxytremorine


 T+,
T+, C
C


