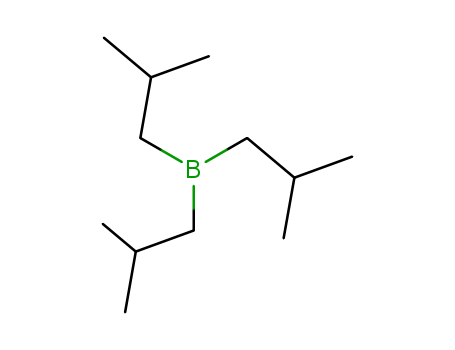
- Chemical Name:Triisobutylborane
- CAS No.:1116-39-8
- Molecular Formula:C12H27 B
- Molecular Weight:182.157
- Hs Code.:2931900090
- European Community (EC) Number:214-235-3
- DSSTox Substance ID:DTXSID3061500
- Nikkaji Number:J203.336D
- Wikidata:Q81989504
- Mol file:1116-39-8.mol
Synonyms:Triisobutylborane;1116-39-8;tris(2-methylpropyl)borane;Borane, tris(2-methylpropyl)-;EINECS 214-235-3;triisobutylboron;DTXSID3061500;XDSSGQHOYWGIKC-UHFFFAOYSA-


 F:Highly flammable;
F:Highly flammable;

