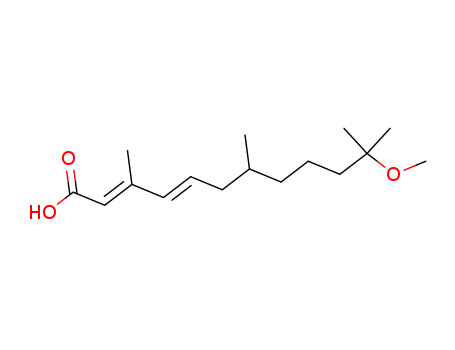
- Chemical Name:(2E,4E)-11-Methoxy-3,7,11-trimethyldodeca-2,4-dienoic acid
- CAS No.:53092-52-7
- Deprecated CAS:40596-67-6
- Molecular Formula:C16H28 O3
- Molecular Weight:268.397
- Hs Code.:2918990090
- European Community (EC) Number:258-355-4
- UNII:MB9LR353F8
- DSSTox Substance ID:DTXSID10886034
- Wikidata:Q27085172
- Pharos Ligand ID:9K9JN8YLLBPZ
- ChEMBL ID:CHEMBL289635
- Mol file:53092-52-7.mol
Synonyms:Methoprene acid;53092-52-7;2,4-Dodecadienoic acid, 11-methoxy-3,7,11-trimethyl-, (2E,4E)-;ZR-725;UNII-MB9LR353F8;(2E,4E)-11-METHOXY-3,7,11-TRIMETHYLDODECA-2,4-DIENOIC ACID;MB9LR353F8;EINECS 258-355-4;(+-)-(2E,4E)-11-Methoxy-3,7,11-trimethyl-2,4-dodecadienoic acid;C16H28O3;S Methoprene acid;Spectrum5_001945;D0CT1F;BSPBio_001416;BML2-E11;CHEMBL289635;GTPL2812;SCHEMBL3052148;SCHEMBL3052151;(2E,4E)-(+-)-11-methoxy-3,7,11-trimethyldodeca-2,4-dienoic acid;2,4-Dodecadienoic acid, 11-methoxy-3,7,11-trimethyl-, (E,E)-(.+.)-;CHEBI:91685;DTXSID10886034;HMS1361G18;HMS1791G18;HMS1989G18;HMS3402G18;Methoprene acid, >=98% (TLC);AKOS037645613;AS-6176;IDI1_033886;NCGC00161329-01;NCGC00161329-02;NCGC00161329-03;NCGC00161329-04;BRD-A41145729-001-02-7;Q27085172;(2E,4E)-(1)-11-Methoxy-3,7,11-trimethyldodeca-2,4-dienoic acid;2,4-DODECADIENOIC ACID, 11-METHOXY-3,7,11-TRIMETHYL-, (E,E)-;TRANS,TRANS-11-METHOXY-3,7,11-TRIMETHYL-2,4-DODECADIENOIC ACID;2,4-DODECADIENOIC ACID, 11-METHOXY-3,7,11-TRIMETHYL-, (E,E)-(+/-)-



 Xi
Xi

