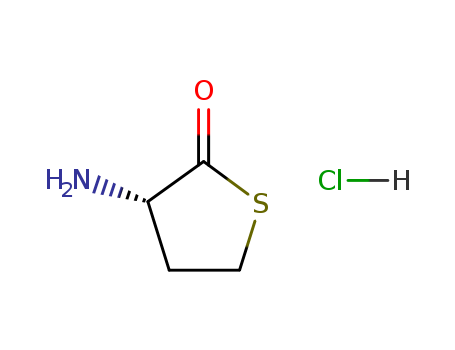
- Chemical Name:(S)-3-Aminodihydrothiophen-2(3H)-one hydrochloride
- CAS No.:31828-68-9
- Molecular Formula:C4H7NOS.ClH
- Molecular Weight:153.633
- Hs Code.:2934999090
- European Community (EC) Number:250-824-1
- UNII:2HTL9Q12FE
- DSSTox Substance ID:DTXSID60953745
- Wikidata:Q27254763
- Mol file:31828-68-9.mol
Synonyms:31828-68-9;L-Homocysteine thiolactone hydrochloride;(S)-3-Aminodihydrothiophen-2(3H)-one hydrochloride;(S)-3-Aminodihydrothiophen-2(3H)-one HCl;Homocysteine thiolactone hydrochloride, L-;UNII-2HTL9Q12FE;2HTL9Q12FE;(3S)-3-aminothiolan-2-one;hydrochloride;L-Homocysteine thiolactone (hydrochloride);L-homocysteine thiolactone HCl;EINECS 250-824-1;L-2-Amino-4-mercaptobutyric acid 1,4-thiolactone hydrochloride;C4-H7-N-O-S.Cl-H;SCHEMBL185765;DTXSID60953745;ZSEGSUBKDDEALH-DFWYDOINSA-N;AKOS015950984;CS-6394;HY-101404A;AS-49937;PD103002;O10218;3-Aminothiolan-2-one--hydrogen chloride (1/1);A919235;HOMOCYSTEINE THIOLACTONE HYDROCHLORIDE, (+)-;Q27254763;L-Homocysteine thiolactone hydrochloride, >=98% (TLC);Z5069354967;HOMOCYSTEINE L-FORM THIOLACTONE HYDROCHLORIDE [MI];L-Homocysteine thiolactone hydrochloride, >=99.0% (TLC);2(3H)-THIOPHENONE, 3-AMINODIHYDRO-, HYDROCHLORIDE (1:1), (3S)-