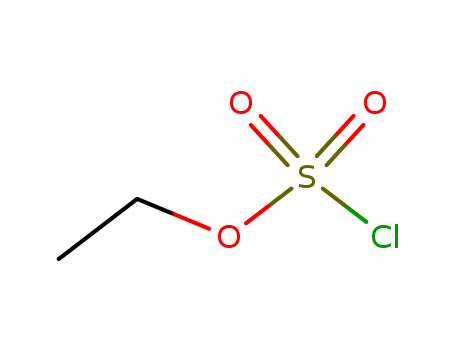
- Chemical Name:Chlorosulfuric acid, ethyl ester
- CAS No.:625-01-4
- Molecular Formula:C2H5 Cl O3 S
- Molecular Weight:144.579
- Hs Code.:2905199090
- European Community (EC) Number:210-874-7
- UNII:RB66T45JQP
- DSSTox Substance ID:DTXSID8060797
- Nikkaji Number:J297.799K
- Wikidata:Q9189978
- Mol file:625-01-4.mol
Synonyms:625-01-4;Ethyl chlorosulfonate;Ethyl chlorosulfate;Chlorosulfuric acid, ethyl ester;chlorosulfonyloxyethane;ethyl sulfurochloridate;Ethyl chlorosulphonate;Ethoxysulfonyl chloride;RB66T45JQP;Chlorosulfuric acid ethyl ester;EINECS 210-874-7;ethylsulfurochloridate;Chlorsulfonsaureathylester;ETHYL CHLOROSULPHATE;UNII-RB66T45JQP;SCHEMBL220237;DTXSID8060797;MFCD00152491;AKOS006273878;FD10603;CHLOROSULPHURIC ACID, ETHYL ESTER;FT-0696696;Q9189978