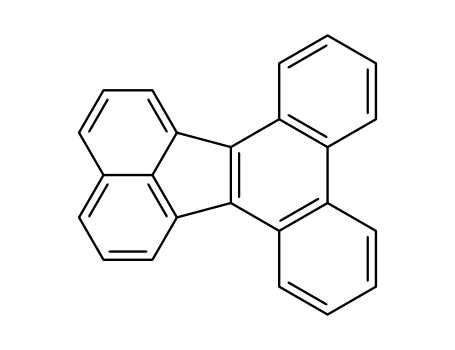
- Chemical Name:Dibenzo[j,l]fluoranthene
- CAS No.:203-18-9
- Molecular Formula:C24H14
- Molecular Weight:302.38
- Hs Code.:2902909090
- DSSTox Substance ID:DTXSID30174182
- Nikkaji Number:J270.046H
- Wikidata:Q83044244
- Mol file:203-18-9.mol
Synonyms:Dibenzo[j,l]fluoranthene;Dibenzo(j,l)fluoranthene;203-18-9;CCRIS 8725;hexacyclo[14.7.1.02,15.03,8.09,14.020,24]tetracosa-1(23),2(15),3,5,7,9,11,13,16,18,20(24),21-dodecaene;Dibenzo[j,I]fluoranthene;DTXSID30174182;JYVYSXOKKXPQHQ-UHFFFAOYSA-N;LS-61035