E.F.K. Achazi et al. / Journal of Controlled Release xxx (2014) xxx–xxx
9
[7] R. Haag, Supramolecular drug-delivery systems based on polymeric core–shell 618
architectures, Angew. Chem. Int. Ed. 43 (2004) 278–282. 619
[8] A.X. Mahmud, X. B., H.M. Aliabadi, A. Lavasanifar, Polymeric micelles for drug 6Q230
targeting, J. Drug Target. 15 (2007) 553–584. 621
[9] K. Miyata, R.J. Christie, K. Kataoka, Polymeric micelles for nano-scale drug delivery, 622
React. Funct. Polym. 71 (2011) 227–234. 623
[10] V.P. Torchilin, Recent advances with liposomes as pharmaceutical carriers, Nat. Rev. 624
Drug Discov. 4 (2005) 145–160. 625
[11] R.P. Brinkhuis, F.P.J.T. Rutjes, J.C.M. van Hest, Polymeric vesicles in biomedical 626
applications, Polym. Chem. 2 (2011) 1449–1462. 627
[12] C.S. Popeney, M.C. Lukowiak, C. Böttcher, B. Schade, P. Welker, D. Mangoldt, G. 628
Gunkel, Z. Guan, R. Haag, Tandem coordination, ring-opening, hyperbranched poly- 629
merization for the synthesis of water-soluble core–shell unimolecular transporters, 630
ACS Macro Lett. 1 (2012) 564–567.
631
[13] M.R. Radowski, A. Shukla, H. von Berlepsch, C. Boettcher, G. Pickaert, H. Rehage, R. 632
Haag, Supramolecular aggregates of dendritic multishell architectures as universal 633
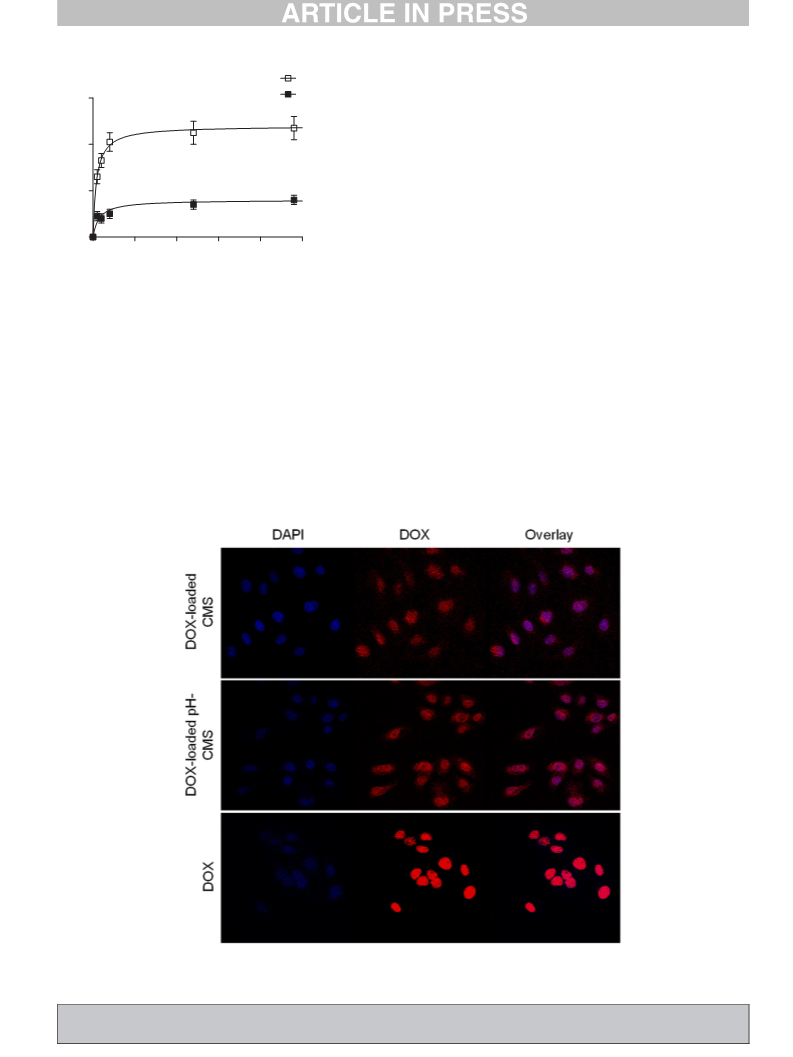
Fig. 7. DOX-loaded pH-responsive CMS nanocarriers get cleaved due to a drop in the pH
inside of the late endosome or lysosome after cellular uptake. The transported DOX gets
released and can induce cell death.
nanocarriers, Angew. Chem. Int. Ed. 46 (2007) 1265–1269.
634
[14] E. Fleige, B. Ziem, M. Grabolle, R. Haag, U. Resch-Genger, Aggregation phenomena of 635
host and guest upon the loading of dendritic core–multishell nanoparticles with 636
solvatochromic dyes, Macromolecules 45 (2012) 9452–9459.
[15] T. Etrych, J. Strohalm, P. Chytil, P. Černoch, L. Starovoytova, M. Pechar, K. Ulbrich, 638
Biodegradable star HPMA polymer conjugates of doxorubicin for passive tumor 639
targeting, Eur. J. Pharm. Sci. 42 (2011) 527–539.
[16] T. Etrych, L. Kovář, J. Strohalm, P. Chytil, B. Říhová, K. Ulbrich, Biodegradable star 641
HPMA polymer-drug conjugates: biodegradability, distribution and anti-tumor 642
efficacy, J. Control. Release 154 (2011) 241–248.
[17] M. Calderón, P. Welker, K. Licha, I. Fichtner, R. Graeser, R. Haag, F. Kratz, Develop- 644
637
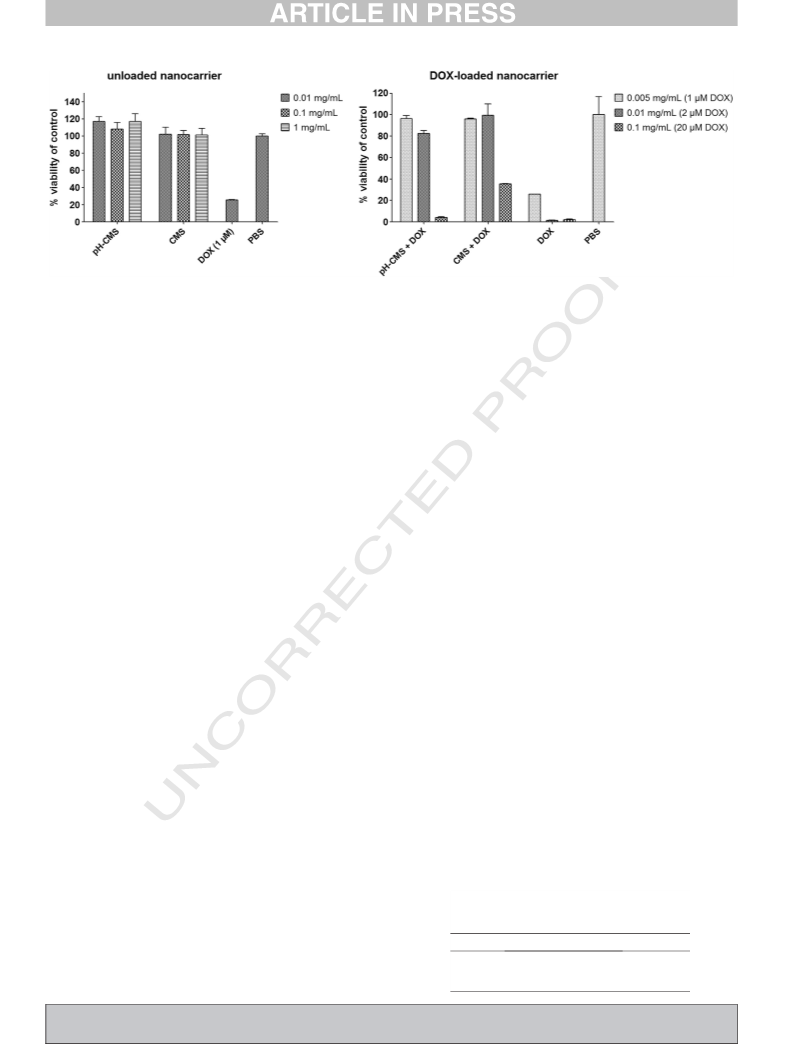
572 them in Table 5. The pH-CMS nanocarriers showed an IC50 value half as
573 high as the CMS nanocarriers. The enhanced toxicity of the pH-cleavable
574 CMS nanocarriers proves them to be superior over the pH-stable CMS
575 system as it was able to actively release the encapsulated guest upon a
576 pH-external stimulus, equivalent to the pH in the endosomal compart-
577 ments [46] after cellular uptake (see Fig. 7).
640
643
ment of efficient acid cleavable multifunctional prodrugs derived from dendritic 645
OOF
polyglycerol with a poly(ethylene glycol) shell, J. Control. Release 151 (2011) 646
295–301.
578 4. Conclusion
647
[18] M.A. Quadir, M.R. Radowski, F. Kratz, K. Licha, P. Hauff, R. Haag, Dendritic 648
multishell architectures for drug and dye transport, J. Control. Release 132 (2008) 649
579
580
581
582
583
584
585
586
587
588
589
590
591
592
593
594
595
596
597
The introduction of the aromatic imine linkage into the CMS
nanocarriers enabled the already highly versatile CMS nanocarriers to be-
come pH-responsive. The imine linkage used was rapidly cleaved at a pH
of 5 and lower. Doxorubicin-loaded pH-responsive CMS nanocarriers
were stable at pH 7.4 and did not show any release. By real time cell
analysis we were able to demonstrate that pH-responsive nanocarriers
(pH-CMS) could release doxorubicin more efficiently under the acidic
conditions of intracellular compartments and therefore showed higher
cytotoxicity in comparison to the stable CMS nanocarrier. Interestingly,
the transport of doxorubicin was achieved by unimolecular CMS and
pH-CMS nanocarriers and not in aggregates as it was observed for
other guest molecules, such as Nile red. This also resulted in higher trans-
port capacities of up to 5 wt.-% for doxorubicin as compared to 0.3 wt.-%
for Nile red. Hence, the pH-responsive CMS nanocarriers are highly po-
tent unimolecular drug delivery systems. Due to their size, they should
benefit from the EPR effect and can actively release their payload inside
tumors.
289–294.
650
[19] C. Treiber, M.A. Quadir, P. Voigt, M. Radowski, S. Xu, L.-M. Munter, T.A. Bayer, M. 651
Schaefer, R. Haag, G. Multhaup, Cellular copper import by nanocarrier systems, in- 652
tracellular availability, and effects on amyloid beta peptide secretion, Biochemistry 653
48 (2009) 4273–4284.
654
[20] H. Maeda, K. Greish, J. Fang, The EPR effect and polymeric drugs: a paradigm shift for 655
cancer chemotherapy in the 21st century, in: R. Satchi-Fainaro, R. Duncan (Eds.), 656
Polymer Therapeutics II, Springer, Berlin Heidelberg, 2006, pp. 103–121.
657
[21] H. Maeda, H. Nakamura, J. Fang, The EPR effect for macromolecular drug delivery to 658
solid tumors: improvement of tumor uptake, lowering of systemic toxicity, and 659
distinct tumor imaging in vivo, Adv. Drug Deliv. Rev. 65 (2013) 71–79.
660
[22] S. Kuechler, M. Abdel-Mottaleb, A. Lamprecht, M.R. Radowski, R. Haag, M. Schaefer- 661
Korting, Influence of nanocarrier type and size on skin delivery of hydrophilic 662
agents, Int. J. Pharm. 377 (2009) 169–172.
663
[23] S. Kuechler, M.R. Radowski, T. Blaschke, M. Dathe, J. Plendl, R. Haag, M. Schaefer- 664
Korting, K.D. Kramer, Nanoparticles for skin penetration enhancement — a compar- 665
ison of a dendritic core–multishell-nanotransporter and solid lipid nanoparticles, 666
Eur. J. Pharm. Biopharm. 71 (2009) 243–250.
667
[24] S.F. Haag, E. Fleige, M. Chen, A. Fahr, C. Teutloff, R. Bittl, J. Lademann, M. Schäfer- 668
Korting, R. Haag, M.C. Meinke, Skin penetration enhancement of core–multishell 669
nanotransporters and invasomes measured by electron paramagnetic resonance 670
spectroscopy, Int. J. Pharm. 416 (2011) 223–228.
671
Funding sources
[25] E. Fleige, M.A. Quadir, R. Haag, Stimuli-responsive polymeric nanocarriers for the 672
controlled transport of active compounds: concepts and applications, Adv. Drug 673
The authors would like to thank the focus area nanoscale of the Freie
Deliv. Rev. 64 (2012) 866–884.
674
598 Universität Berlin and the SFB 1112 for the financial support.
[26] P. Vaupel, F. Kallinowski, P. Okunieff, Blood flow, oxygen and nutrient supply, and 675
metabolic microenvironment of human tumors: a review, Cancer Res. 49 (1989) 676
599 Acknowledgment
6449–6465.
[27] S. Mukherjee, R.N. Ghosh, F.R. Maxfield, Endocytosis, Physiol. Rev. 77 (1997) 678
759–803. 679
677
600
We are grateful to Dr. Juliane Keilitz and Dr. Pamela Winchester for
[28] E.S. Lee, Z. Gao, D. Kim, K. Park, I.C. Kwon, Y.H. Bae, Super pH-sensitive multifunc- 680
tional polymeric micelle for tumor pHe specific TAT exposure and multidrug 681
601 proofreading the manuscript.
resistance, J. Control. Release 129 (2008) 228–236.
682
[29] Y. Bae, K. Kataoka, Intelligent polymeric micelles from functional poly(ethylene 683
glycol)-poly(amino acid) block copolymers, Adv. Drug Deliv. Rev. 61 (2009) 684
602 Appendix A. Supplementary data
768–784.
[30] F. Meng, Z. Zhong, J. Feijen, Stimuli-responsive polymersomes for programmed drug 686
delivery, Biomacromolecules 10 (2009) 197–209. 687
[31] U. Borchert, U. Lipprandt, M. Bilang, A. Kimpfler, A. Rank, R. Peschka-Süss, R. 688
Schubert, P. Lindner, S. Förster, pH-Induced release from P2VP–PEO block copoly- 689
mer vesicles, Langmuir 22 (2006) 5843–5847. 690
[32] S. Xu, M. Krämer, R. Haag, pH-Responsive dendritic core–shell architectures as 691
amphiphilic nanocarriers for polar drugs, J. Drug Target. 14 (2006) 367–374. 692
685
603
Supplementary data to this article can be found online at http://dx.
605 References
606
607
608
609
610
611
612
613
614
615
616
617
[1] R. Haag, F. Kratz, Polymer therapeutics: concepts and applications, Angew. Chem.
Int. Ed. 45 (2006) 1198–1215.
[2] R. Duncan, M.J. Vicent, Polymer therapeutics-prospects for 21st century: the end of
the beginning, Adv. Drug Deliv. Rev. 65 (2013) 60–70.
[3] M.E. Davis, Z. Chen, D.M. Shin, Nanoparticle therapeutics: an emerging treatment
modality for cancer, Nat. Rev. Drug Discov. 7 (2008) 771–782.
[4] J. Kopeček, Polymer-drug conjugates: origins, progress to date and future directions,
Adv. Drug Deliv. Rev. 65 (2013) 49–59.
[5] R. Duncan, Polymer therapeutics as nanomedicines: new perspectives, Curr. Opin.
Biotechnol. 22 (2011) 492–501.
[33] S. Xu, Y. Luo, R. Haag, Water-soluble pH-responsive dendritic core–shell 693
nanocarriers for polar dyes based on poly(ethylene imine), Macromol. Biosci. 7 694
(2007) 968–974.
[34] S. Xu, Y. Luo, R. Haag, Structure–transport relationship of dendritic core–shell 696
nanocarriers for polar dyes, Macromol. Rapid Commun. 29 (2008) 171–174. 697
695
[35] S. Xu, Y. Luo, R. Graeser, A. Warnecke, F. Kratz, P. Hauff, K. Licha, R. Haag, Develop- 698
ment of pH-responsive core–shell nanocarriers for delivery of therapeutic and 699
diagnostic agents, Bioorg. Med. Chem. Lett. 19 (2009) 1030–1034.
700
[36] M. Krämer, J.-F. Stumbé, H. Türk, S. Krause, A. Komp, L. Delineau, S. Prokhorova, H. 701
Kautz, R. Haag, pH-Responsive molecular nanocarriers based on dendritic core– 702
[6] F. Kratz, I.A. Müller, C. Ryppa, A. Warnecke, Prodrug strategies in anticancer chemo-
therapy, ChemMedChem 3 (2008) 20–53.
shell architectures, Angew. Chem. Int. Ed. 41 (2002) 4252–4256.
703
Please cite this article as: E.F.K. Achazi, et al., pH-responsive dendritic core–multishell nanocarriers, J. Control. Release (2014), http://dx.doi.org/

 Fleige, Emanuel
Fleige, Emanuel
 Achazi, Katharina
Achazi, Katharina
 Schaletzki, Karolina
Schaletzki, Karolina
 Triemer, Therese
Triemer, Therese
 Haag, Rainer
Haag, Rainer