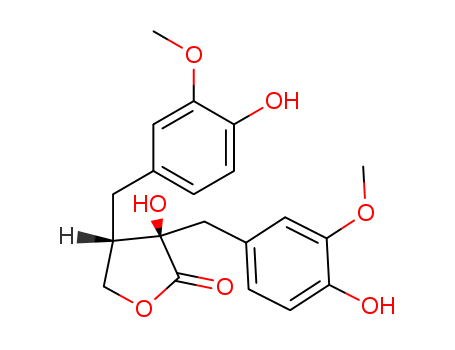
- Chemical Name:Epinortrachelogenin
- CAS No.:125072-69-7
- Molecular Formula:C20H22 O7
- Molecular Weight:374.39
- Hs Code.:
- DSSTox Substance ID:DTXSID501130659
- Nikkaji Number:J607.701C
- Wikidata:Q104399308
- Mol file:125072-69-7.mol
Synonyms:Epinortrachelogenin;125072-69-7;(3R,4S)-3-hydroxy-3,4-bis[(4-hydroxy-3-methoxyphenyl)methyl]oxolan-2-one;DTXSID501130659;HY-N3832;AKOS040761688;FS-8005;CS-0024295;(3R,4S)-Dihydro-3-hydroxy-3,4-bis[(4-hydroxy-3-methoxyphenyl)methyl]-2(3H)-furanone;2(3H)-Furanone, dihydro-3-hydroxy-3,4-bis[(4-hydroxy-3-methoxyphenyl)methyl]-, (3R,4S)-