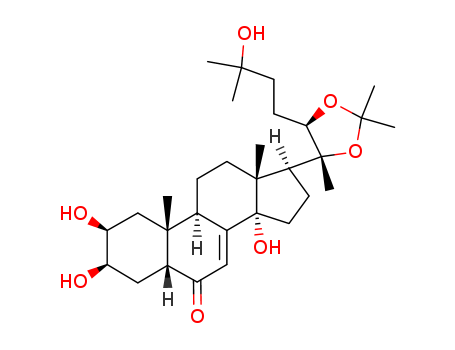
- Chemical Name:Ecdysterone 20,22-monoacetonide
- CAS No.:22798-96-5
- Molecular Formula:C30H48O7
- Molecular Weight:520.707
- Hs Code.:
- DSSTox Substance ID:DTXSID501346563
- Nikkaji Number:J416.586A
- Wikidata:Q105023223
- ChEMBL ID:CHEMBL2087536
- Mol file:22798-96-5.mol
Synonyms:Ecdysterone 20,22-monoacetonide;22798-96-5;20-Hydroxyecdysone 20,22-acetonide;CHEMBL2087536;DTXSID501346563;STL564977;AKOS037623238;FS-9622;(22R)-2beta,3beta,14,25-Tetrahydroxy-20,22-(isopropylidenedioxy)-5beta-cholest-7-en-6-one;(2beta,3beta,5beta,17beta)-2,3,14-trihydroxy-17-[(4R,5R)-5-(3-hydroxy-3-methylbutyl)-2,2,4-trimethyl-1,3-dioxolan-4-yl]androst-7-en-6-one