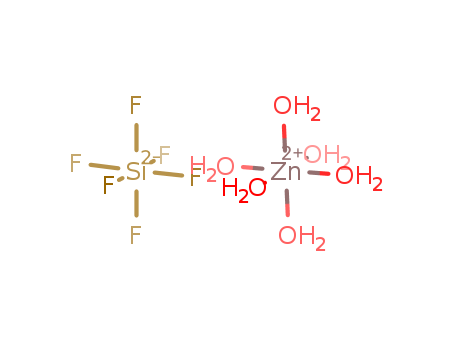
- Chemical Name:Zinc silicofluoride
- CAS No.:18433-42-6
- Molecular Formula:F6Si*H12O6Zn
- Molecular Weight:315.558
- Hs Code.:
- Mol file:18433-42-6.mol
Synonyms:Silicate(2-),hexafluoro-, zinc (1:1), hexahydrate (9CI);Silicate(2-), hexafluoro-, zinc,hexahydrate (8CI);Zinc fluorosilicate (ZnSiF6), hexahydrate;Zinchexafluorosilane hexahydrate;Zinc hexafluorosilicate (ZnSiF6), hexahydrate;Zinc hexafluorosilicate hexahydrate;Zinc hexafluorosilicate(2-) hexahydrate;Zinc silicon fluoride (ZnSiF6), hexahydrate;


 C
C


