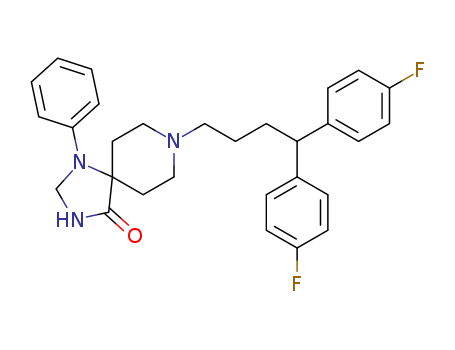
- Chemical Name:Fluspirilene
- CAS No.:1841-19-6
- Molecular Formula:C29H31 F2 N3 O
- Molecular Weight:475.581
- Hs Code.:2933990090
- European Community (EC) Number:217-418-6
- UNII:C5QA4GLR9M
- DSSTox Substance ID:DTXSID7045152
- Nikkaji Number:J7.791G
- Wikipedia:Fluspirilene
- Wikidata:Q408300
- NCI Thesaurus Code:C171733
- Pharos Ligand ID:P7G2PKY655TM
- Metabolomics Workbench ID:43630
- ChEMBL ID:CHEMBL46516
- Mol file:1841-19-6.mol
Synonyms:Beta, Fluspirilen;Fluspi;Fluspirilen beta;Fluspirilen Lindo;Fluspirilene;Imap;kivat;Redeptin;Spirodiflamine