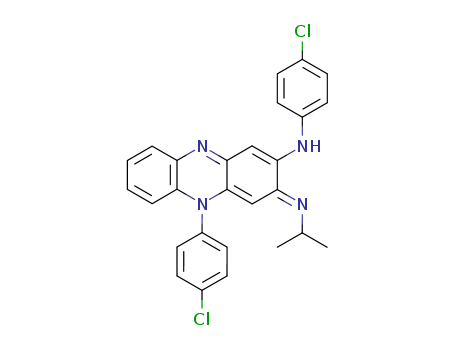
- Chemical Name:Clofazimine
- CAS No.:2030-63-9
- Molecular Formula:C27H22Cl2N4
- Molecular Weight:473.404
- Hs Code.:35040000
- European Community (EC) Number:217-980-2
- NSC Number:759283,141046
- UNII:D959AE5USF
- DSSTox Substance ID:DTXSID7022839
- Nikkaji Number:J9.543E
- Wikipedia:Clofazimine
- Wikidata:Q418611,Q105302601
- NCI Thesaurus Code:C47456
- Pharos Ligand ID:GKXYGK77W4KM
- Metabolomics Workbench ID:49989
- ChEMBL ID:CHEMBL1292,CHEMBL1083384
- Mol file:2030-63-9.mol
Synonyms:B 663;B-663;B663;Clofazimine;G 30,320;G-30,320;G30,320;Lamprene;N,5-Bis(4-chlorophenyl)-3,5-dihydro-3-((1-methylethyl)imino)-2-phenazinamine


 Xn,
Xn,  Xi
Xi


