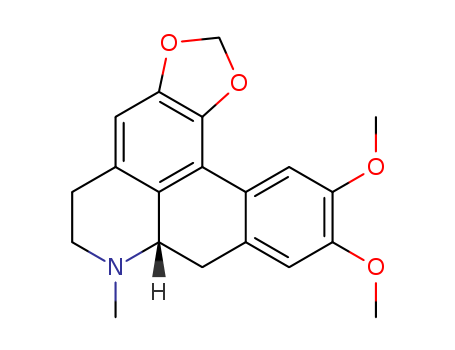
- Chemical Name:Dicentrine
- CAS No.:517-66-8
- Molecular Formula:C20H21 N O4
- Molecular Weight:339.391
- Hs Code.:
- NSC Number:406035
- UNII:J2ZGT5M0N7
- DSSTox Substance ID:DTXSID10199651
- Nikkaji Number:J9.429C
- Wikipedia:Dicentrine
- Wikidata:Q15410930
- Metabolomics Workbench ID:136649
- ChEMBL ID:CHEMBL464748
- Mol file:517-66-8.mol
Synonyms:(R)-(-)-dicentrine;dicentrine;dicentrine nitrate, (S)-isomer;dicentrine, (-)-;dicentrine, (R)-isomer;L-dicentrine