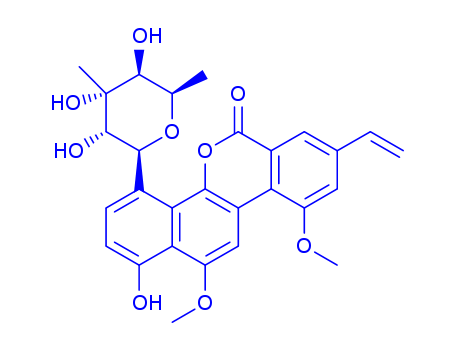
- Chemical Name:Albacarcin V
- CAS No.:82196-88-1
- Molecular Formula:C28H28O9
- Molecular Weight:508.525
- Hs Code.:
- NSC Number:613946,354844
- DSSTox Substance ID:DTXSID20918931
- ChEMBL ID:CHEMBL1728866
- Mol file:82196-88-1.mol
Synonyms:Albacarcin V;NSC354844;MLS002701849;92841-46-8;8-ethenyl-1-hydroxy-10,12-dimethoxy-4-(3,4,5-trihydroxy-4,6-dimethyloxan-2-yl)naphtho[1,2-c]isochromen-6-one;8-ethenyl-1-hydroxy-10,12-dimethoxy-4-[(2R,3R,4S,5R,6S)-3,4,5-trihydroxy-4,6-dimethyloxan-2-yl]naphtho[1,2-c]isochromen-6-one;NSC 354844;VIRENOMYCIN V;CHEMBL1728866;DTXSID20918931;OMDANBMKOUVKAG-UHFFFAOYSA-N;NSC613946;NSC-354844;NSC-613946;6H-Benzo(d)naphtho(1,2-b)pyran-6-one, 4-(6-deoxy-3-C-methyl-beta-L-gulopyranosyl)-8-ethenyl-1-hydroxy-10,12-dimethoxy-;NCI60_003193;SMR001565441;1,5-Anhydro-6-deoxy-1-(8-ethenyl-1-hydroxy-10,12-dimethoxy-6-oxo-6H-benzo[d]naphtho[1,2-b]pyran-4-yl)-3-C-methylhexitol;4-(6-Deoxy-3-C-methyl-.beta.-gulopyranosyl)-8-vinyl-1-hydroxy-10,12-dimethoxy-6H-benzo[d]naphtho[1,2-b]pyran-6-one (chrysomycin A);6H-Benzo[d]naphtho[1, 4-(6-deoxy-3-C-methyl-.beta.-D-gulopyranosyl)-8-ethenyl-1-hydroxy-10,12-dimethoxy-;6H-Benzo[d]naphtho[1,4-(6-deoxy-3-C-methyl-.beta.-D-gulopyranosyl-8-ethenyl-1-hydroxy-10,12-dimethoxy-