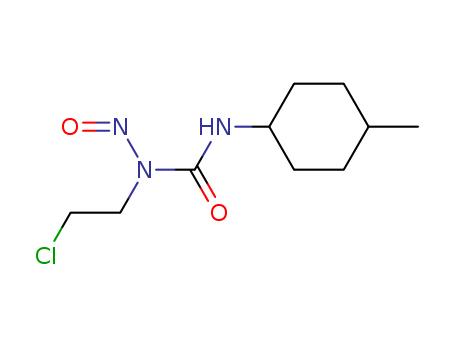
- Chemical Name:Semustine
- CAS No.:13909-09-6
- Deprecated CAS:56748-54-0
- Molecular Formula:C10H18ClN3O2
- Molecular Weight:247.725
- Hs Code.:2924299090
- European Community (EC) Number:634-275-9
- NSC Number:758471,135091,95441
- UN Number:3249
- UNII:2281H4FBL9,EGU4CMI14D
- DSSTox Substance ID:DTXSID8031603,DTXSID301170045
- Nikkaji Number:J60.486K,J83.888H,J8.456E
- Wikipedia:Semustine
- Wikidata:Q1230937,Q27277177
- NCI Thesaurus Code:C827
- Metabolomics Workbench ID:67496
- ChEMBL ID:CHEMBL12948,CHEMBL1967746,CHEMBL2051944
- Mol file:13909-09-6.mol
Synonyms:Me CCNU;Me-CCNU;MeCCNU;Methyl CCNU;Methyl-CCNU;NSC 95441;NSC-95441;NSC95441;Semustine


 T
T


