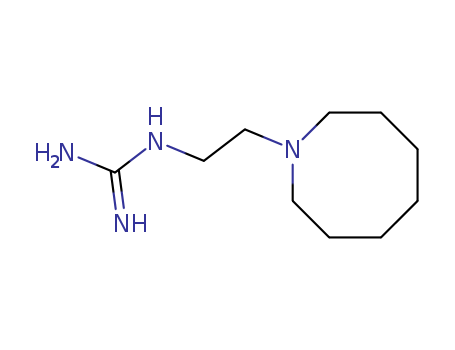
- Chemical Name:Guanethidine
- CAS No.:55-65-2
- Molecular Formula:C10H22 N4
- Molecular Weight:198.311
- Hs Code.:
- European Community (EC) Number:200-241-3
- UNII:ZTI6C33Q2Q
- DSSTox Substance ID:DTXSID5023116
- Nikkaji Number:J4.562D
- Wikipedia:Guanethidine
- Wikidata:Q420673
- NCI Thesaurus Code:C65830
- Pharos Ligand ID:XSVDCUPXXRUS
- Metabolomics Workbench ID:43388
- ChEMBL ID:CHEMBL765
- Mol file:55-65-2.mol
Synonyms:((2-Hexahydro-1(2H)-azocinyl)ethyl)guanidine;Guanethidine;Guanethidine Monosulfate;Guanethidine Sulfate;Guanethidine Sulfate (1:1);Guanethidine Sulfate (1:2);Guanethidine Sulfate (2:1);Guanethidine Sulfate (2:1), 14C-Labeled;Ismelin;Isobarin;Monosulfate, Guanethidine;Octadine;Oktadin;Sulfate, Guanethidine