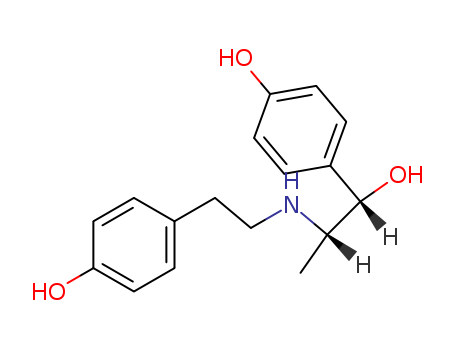
- Chemical Name:Ritodrine
- CAS No.:26652-09-5
- Molecular Formula:C17H21NO3
- Molecular Weight:287.359
- Hs Code.:
- European Community (EC) Number:247-879-9
- DSSTox Substance ID:DTXSID7048534
- Nikkaji Number:J239.135J
- Wikipedia:Ritodrine
- Wikidata:Q5577596
- NCI Thesaurus Code:C61929
- Pharos Ligand ID:AVRXD3GMWW12
- Metabolomics Workbench ID:43143
- ChEMBL ID:CHEMBL785
- Mol file:26652-09-5.mol
Synonyms:DU 21220;DU-21220;DU21220;Hydrochloride, Ritodrine;Pre Par;Pre-Par;PrePar;Ritodrine;Ritodrine Hydrochloride;Yutopar