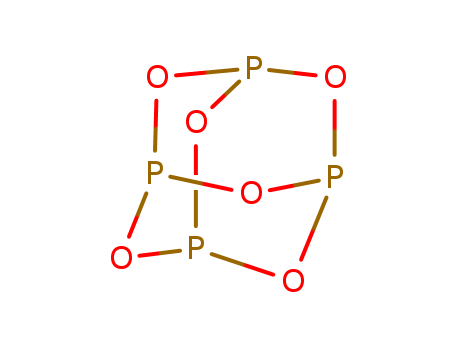
- Chemical Name:Phosphorus oxide
- CAS No.:12440-00-5
- Molecular Formula:O6P4
- Molecular Weight:219.891
- Hs Code.:
- European Community (EC) Number:235-670-5
- DSSTox Substance ID:DTXSID80907588
- Wikipedia:Phosphorus_trioxide
- Wikidata:Q411876
- Mol file:12440-00-5.mol
Synonyms:Tetraphosphorus hexaoxide;P4O6;12440-00-5;10248-58-5;EINECS 235-670-5;(P2O3)2;2,4,6,8,9,10-Hexaoxa-1,3,5,7-tetraphosphatricyclo[3.3.1.13,7]decane;2,4,6,8,9,10-Hexaoxa-1,3,5,7-tetraphosphatricyclo(3.3.1.13,7)decane;phosphorus(III) oxide;Phosphortrioxid;Phosphortrioxyd;Phosphor(III)-oxid;Phosphorus oxide (P4O6);O6P4;CHEBI:37372;DTXSID80907588;O6-P4;P4 O6;tricyclo[3.3.1.1(3,7)]tetraphosphoxane;Tricyclo[3.3.1.1~3,7~]tetraphosphoxane;Q411876;2,4,6,8,9,10-hexaoxa-1,3,5,7-tetraphosphatricyclo[3.3.1.1(3,7)]decane