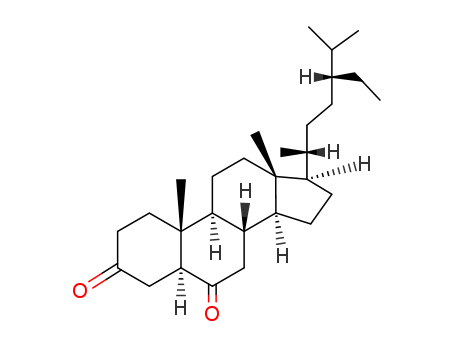
- Chemical Name:5alpha-Stigmastane-3,6-dione
- CAS No.:22149-69-5
- Molecular Formula:C29H48O2
- Molecular Weight:428.699
- Hs Code.:
- UNII:ZJ56JQ4CVD
- DSSTox Substance ID:DTXSID80944796
- Nikkaji Number:J83.551J
- Wikidata:Q72508913
- Mol file:22149-69-5.mol
Synonyms:stigmastane-3,6-dione