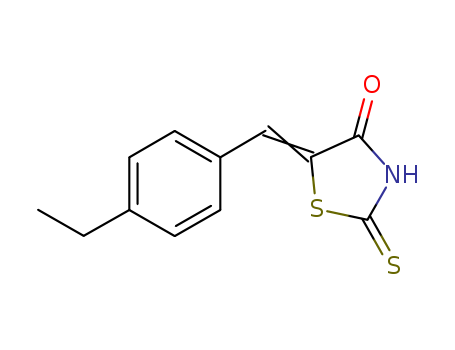
- Chemical Name:(5E)-5-(4-ethylbenzylidene)-2-mercapto-1,3-thiazol-4(5H)-one
- CAS No.:403811-55-2
- Molecular Formula:C12H11NOS2
- Molecular Weight:249.35200
- Hs Code.:2934100090
- DSSTox Substance ID:DTXSID301017239
- Wikidata:Q106041338
- ChEMBL ID:CHEMBL3183888
- Mol file:403811-55-2.mol
Synonyms:c-Myc Inhibitor;(5E)-5-(4-ethylbenzylidene)-2-mercapto-1,3-thiazol-4(5H)-one;5-(4-ethylbenzylidene)-2-sulfanylidene-1,3-thiazolidin-4-one;(5E)-5-[(4-ethylphenyl)methylidene]-2-sulfanylidene-1,3-thiazolidin-4-one;CHEMBL3183888;DTXSID301017239;HMS3653E13;BCP08097;AKOS030690544;SB19479;UPCMLD0ENAT5387305:001;NCGC00185994-02;PD056405;FT-0679472;10058F4;10058 F4


 Xi
Xi


